


生物技术进展 ›› 2024, Vol. 14 ›› Issue (3): 406-412.DOI: 10.19586/j.2095-2341.2023.0174
• 进展评述 • 上一篇
收稿日期:2024-01-02
接受日期:2024-03-25
出版日期:2024-05-25
发布日期:2024-06-18
通讯作者:
武建强
作者简介:李蓓蓓 E-mail: libeibei960129@163.com;
基金资助:Received:2024-01-02
Accepted:2024-03-25
Online:2024-05-25
Published:2024-06-18
Contact:
Jianqiang WU
摘要:
FAS是一种在多种细胞中表达的膜蛋白受体,与其配体FASL结合后激活细胞内下游信号,可导致一种细胞程序性死亡——凋亡。在很多细胞类型中,FAS诱导的凋亡是抑制或杀灭肿瘤细胞的重要因素。近年来发现,在某些细胞微环境条件或肿瘤类型中,FAS可激活与凋亡相反的生物学功能,包括促进细胞的增生和迁移等。但目前对于FAS介导的非凋亡通路的作用和机理尚不完全清楚,与经典凋亡通路的关系也缺乏研究,其在什么条件下、在哪些细胞或肿瘤类型中起作用也有待深入调查。简述了FAS信号在肿瘤细胞中介导的促增生作用,重点总结了其介导非凋亡通路中的几个关键因子以及在临床肿瘤治疗上的应用,以期为肿瘤的临床新治疗方法和新药研究提供思路。
中图分类号:
李蓓蓓, 武建强. 肿瘤细胞中FAS介导的非凋亡信号通路研究进展[J]. 生物技术进展, 2024, 14(3): 406-412.
Beibei LI, Jianqiang WU. Research Progress of Fas-mediated Non-apoptotic Signaling in Tumor Cells[J]. Current Biotechnology, 2024, 14(3): 406-412.
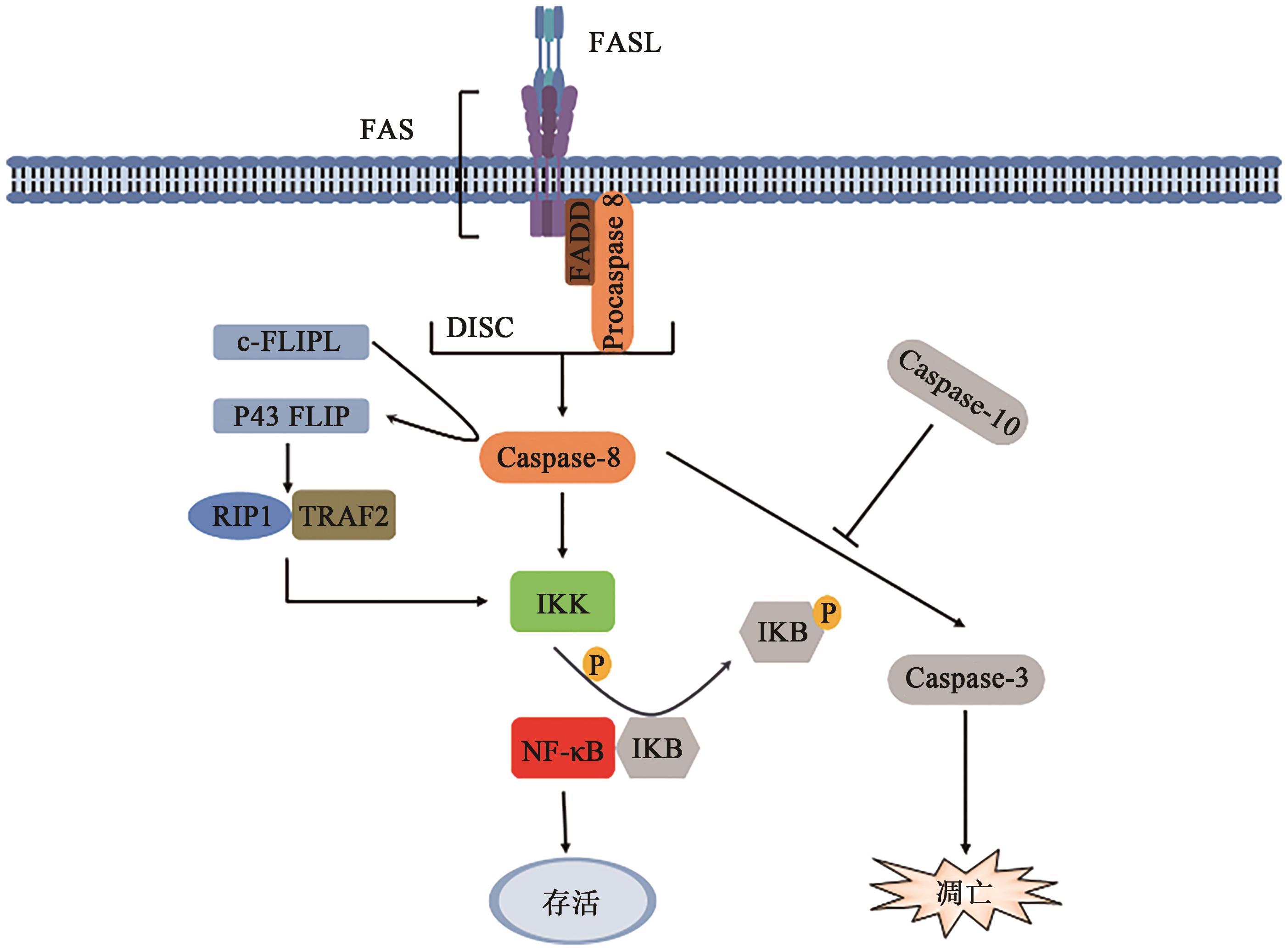
图1 FAS介导的NF-κB通路和凋亡通路注:FADD—FAS死亡结构域相关蛋白;Procaspase—含半胱氨酸的天冬氨酸蛋白酶原;Caspase—含半胱氨酸的天冬氨酸蛋白酶;DISC—凋亡诱导信号复合物;NF-κB—核因子-κB;IKK—核因子-κB抑制物激酶;IKB—核因子-κB蛋白抑制物; RIP1—受体相互作用蛋白1;TRAF2—肿瘤坏死因子受体相关因子2。
Fig. 1 FAS-mediated NF-κB pathway and apoptotic pathway
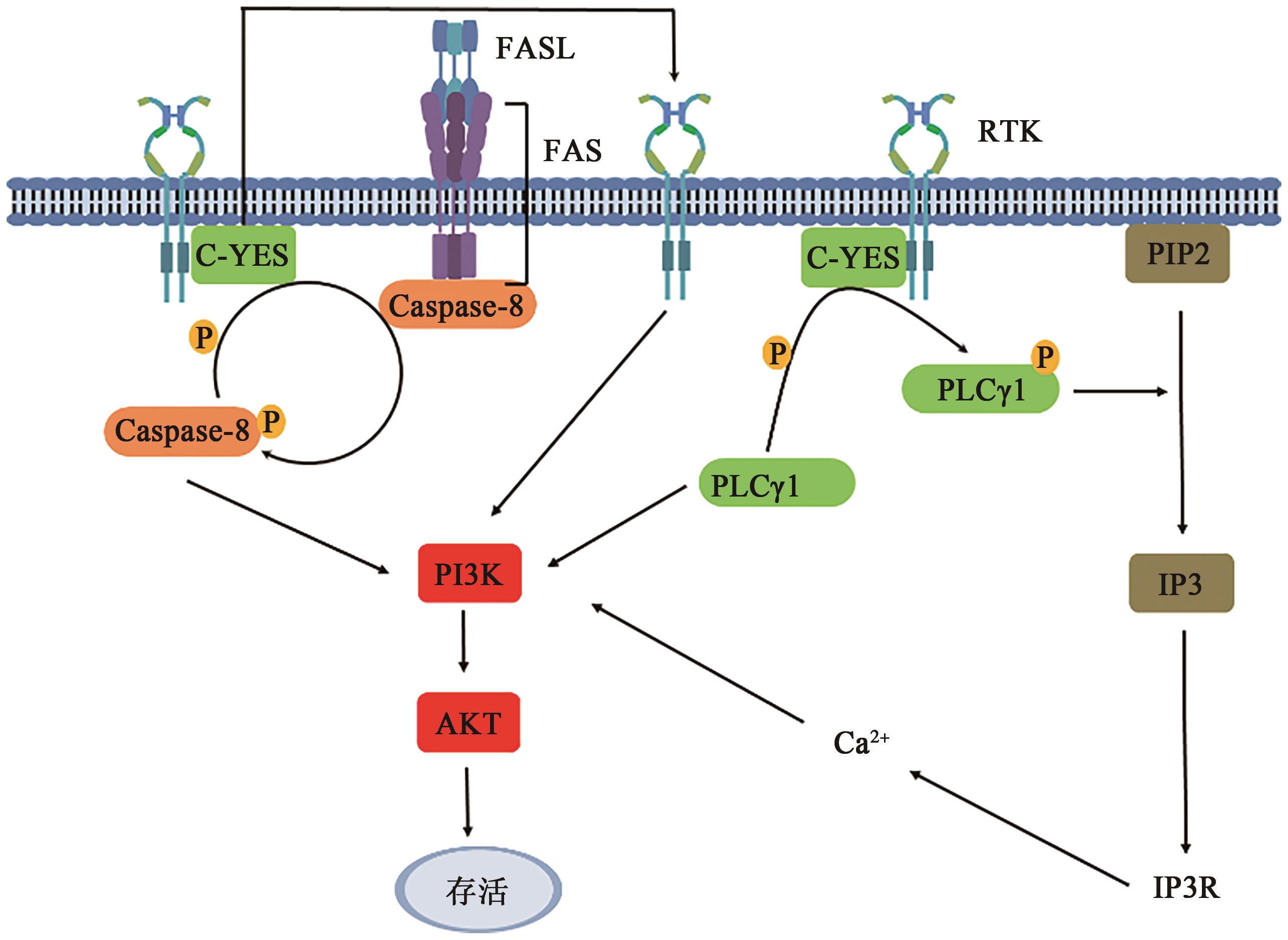
图2 FAS介导的PI3K/AKT通路注:Caspase—含半胱氨酸的天冬氨酸蛋白酶;PI3K—磷脂酰肌醇3-激酶;AKT—蛋白激酶B;PLCγ1—磷脂酶Cγ1;PIP2—磷脂酰肌醇;IP3—肌醇三磷酸;IP3R—肌醇三磷酸受体;DAG—二酰基甘油;RTK—受体酪氨酸激酶。
Fig. 2 FAS-mediated PI3K/AKT pathway

图3 FAS介导的MAPK通路注:FADD—FAS死亡结构域相关蛋白;Procaspase—含半胱氨酸的天冬氨酸蛋白酶原;Caspase—含半胱氨酸的天冬氨酸蛋白酶;EGFR—表皮生长因子受体;ASK1—RAF信号转导激酶-1;MAPK—丝裂原活化蛋白激酶。
Fig. 3 FAS-mediated MAPK pathway
| 1 | SUNG H, FERLAY J, SIEGEL R L, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA-Cancer J. Clin., 2021, 71(3): 209-249. |
| 2 | QIU H, CAO S, XU R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020[J]. Cancer Commun., 2021, 41(10): 1037-1048. |
| 3 | NAGATA S, GOLSTEIN P. The Fas death factor[J]. Science, 1995, 267(5203): 1449-1456. |
| 4 | TIMMER T, DE VRIES E G E, DE JONG S. Fas receptor-mediated apoptosis: a clinical application?[J]. J. Pathol., 2002, 196(2): 125-134. |
| 5 | CHINNAIYAN A M, O'ROURKE K, TEWARI M, et al.. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis[J]. Cell, 1995, 81(4): 505-512. |
| 6 | KISCHKEL F C, HELLBARDT S, BEHRMANN I, et al.. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor[J]. EMBO J., 1995, 14(22): 5579-5588. |
| 7 | FUCHS Y, STELLER H. Programmed cell death in animal development and disease[J]. Cell, 2011, 147(4): 742-758. |
| 8 | NAGATA S, TANAKA M. Programmed cell death and the immune system[J]. Nat. Rev. Immunol., 2017, 17(5): 333-340. |
| 9 | CHEN L, MPARK S, TUMANOV A V, et al.. CD95 promotes tumour growth[J]. Nature, 2010, 465(7297): 492-496. |
| 10 | BARNHART B C, LEGEMBRE P, PIETRAS E, et al.. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells[J]. EMBO J., 2004, 23(15): 3175-3185. |
| 11 | HADJI A, CEPPI P, MURMANN A E, et al.. Death induced by CD95 or CD95 ligand elimination[J]. Cell Rep., 2014, 7(1): 208-222. |
| 12 | QUIJANO-RUBIO C, SILGINER M, WELLER M. CD95 gene deletion may reduce clonogenic growth and invasiveness of human glioblastoma cells in a CD95 ligand-independent manner[J/OL]. Cell Death Discov., 2022, 8(1): 341[2024-04-07]. . |
| 13 | QUIJANO-RUBIO C, SILGINER M, WELLER M. CRISPR/Cas9-mediated abrogation of CD95L/CD95 signaling-induced glioma cell growth and immunosuppression increases survival in murine glioma models[J]. J. Neuro Oncol., 2022, 160(2): 299-310. |
| 14 | CEPPI P, HADJI A, KOHLHAPP F J, et al.. CD95 and CD95L promote and protect cancer stem cells[J/OL]. Nat. Commun., 2014, 5: 5238[2024-04-07]. . |
| 15 | QADIR A S, GUÉGAN J P, GINESTIER C, et al.. CD95/Fas protects triple negative breast cancer from anti-tumor activity of NK cells[J/OL]. iScience, 2021, 24(11): 103348[2024-04-07]. . |
| 16 | BALTA G S, MONZEL C, KLEBER S, et al.. 3D cellular architecture modulates tyrosine kinase activity, thereby switching CD95-mediated apoptosis to survival[J]. Cell Rep., 2019, 29(8): 2295-2306. |
| 17 | LE GALLO M, POISSONNIER A, BLANCO P, et al.. CD95/fas, non-apoptotic signaling pathways, and kinases[J/OL]. Front. Immunol., 2017, 8: 1216[2024-04-07]. . |
| 18 | CHAKRABANDHU K, HUAULT S, DURIVAULT J, et al.. An evolution-guided analysis reveals a multi-signaling regulation of fas by tyrosine phosphorylation and its implication in human cancers[J/OL]. PLoS Biol., 2016, 14(3): e1002401[2024-04-07]. . |
| 19 | LAVRIK I N, GOLKS A, RIESS D, et al.. Analysis of CD95 threshold signaling: triggering of CD95 (FAS/APO-1) at low concentrations primarily results in survival signaling[J]. J. Biol. Chem., 2007, 282(18): 13664-13671. |
| 20 | LEGEMBRE P, BARNHART B C, ZHENG L, et al.. Induction of apoptosis and activation of NF-κB by CD95 require different signalling thresholds[J]. EMBO Rep., 2004, 5(11): 1084-1089. |
| 21 | YU H, LIN L, ZHANG Z, et al.. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study[J/OL]. Signal Transduct. Target. Ther., 2020, 5(1): 209[2024-04-07]. . |
| 22 | NEUMANN L, PFORR C, BEAUDOUIN J, et al.. Dynamics within the CD95 death-inducing signaling complex decide life and death of cells[J/OL]. Mol. Syst. Biol., 2010, 6: 352[2024-04-07]. . |
| 23 | BUCHBINDER J H, PISCHEL D, SUNDMACHER K, et al.. Quantitative single cell analysis uncovers the life/death decision in CD95 network[J/OL]. PLoS Comput. Biol., 2018, 14(9): e1006368[2024-04-07]. . |
| 24 | KREUZ S, SIEGMUND D, RUMPF J J, et al.. NF-κB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP[J]. J. Cell Biol., 2004, 166(3): 369-380. |
| 25 | KARIN M, BEN-NERIAH Y. Phosphorylation meets ubiquitination: the control of NF-κB activity[J]. Annu. Rev. Immunol., 2000, 18: 621-663. |
| 26 | HORN S, HUGHES M A, SCHILLING R, et al.. Caspase-10 negatively regulates caspase-8-mediated cell death, switching the response to CD95L in favor of NF-κB activation and cell survival[J]. Cell Rep., 2017, 19(4): 785-797. |
| 27 | MATSUDA I, MATSUO K, MATSUSHITA Y, et al.. The C-terminal domain of the long form of cellular FLICE-inhibitory protein (c-FLIPL) inhibits the interaction of the caspase 8 prodomain with the receptor-interacting protein 1 (RIP1) death domain and regulates caspase 8-dependent nuclear factor κB (NF-κB) activation[J]. J. Biol. Chem., 2014, 289(7): 3876-3887. |
| 28 | RISSO V, LAFONT E, LE GALLO M. Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases[J/OL]. Cell Death Dis., 2022, 13(3): 248[2024-04-07]. . |
| 29 | FRUMAN D A, CHIU H, HOPKINS B D, et al.. The PI3K pathway in human disease[J]. Cell, 2017, 170(4): 605-635. |
| 30 | FOUQUÉ A, LEGEMBRE P. Study of the CD95-mediated non-apoptotic signaling pathway: PI3K[J]. Methods Mol. Biol., 2017, 1557: 103-110. |
| 31 | TAUZIN S, CHAIGNE-DELALANDE B, SELVA E, et al.. The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway[J/OL]. PLoS Biol., 2011, 9(6): e1001090[2024-04-07]. . |
| 32 | KLEBER S, SANCHO-MARTINEZ I, WIESTLER B, et al.. Yes and PI3K bind CD95 to signal invasion of glioblastoma[J]. Cancer Cell, 2008, 13(3): 235-248. |
| 33 | CURSI S, RUFINI A, STAGNI V, et al.. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression[J]. EMBO J., 2006, 25(9): 1895-1905. |
| 34 | FINLAY D, HOWES A, VUORI K. Critical role for caspase-8 in epidermal growth factor signaling[J]. Cancer Res., 2009, 69(12): 5023-5029. |
| 35 | MOTZ G T, SANTORO S P, WANG L P, et al.. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors[J]. Nat. Med., 2014, 20(6): 607-615. |
| 36 | STELLER E J, RITSMA L, RAATS D A, et al.. The death receptor CD95 activates the cofilin pathway to stimulate tumour cell invasion[J]. EMBO Rep., 2011, 12(9): 931-937. |
| 37 | GUO Y J, PAN W W, LIU S B, et al.. ERK/MAPK signalling pathway and tumorigenesis[J]. Exp. Ther. Med., 2020, 19(3): 1997-2007. |
| 38 | ZHANG Y, JIN T, DOU Z, et al.. The dual role of the CD95 and CD95L signaling pathway in glioblastoma[J/OL]. Front. Immunol., 2022, 13: 1029737[2024-04-07]. . |
| 39 | CHANG H Y, NISHITOH H, YANG X, et al.. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx[J]. Science, 1998, 281(5384): 1860-1863. |
| 40 | KOENIG A, BUSKIEWICZ I A, FORTNER K A, et al.. The c-FLIPL cleavage product p43FLIP promotes activation of extracellular signal-regulated kinase (ERK), nuclear factor κB (NF-κB), and caspase-8 and T cell survival[J]. J. Biol. Chem., 2014, 289(2): 1183-1191. |
| 41 | FARLEY S M, PURDY D E, RYABININA O P, et al.. Fas ligand-induced proinflammatory transcriptional responses in reconstructed human epidermis. Recruitment of the epidermal growth factor receptor and activation of MAP kinases[J]. J. Biol. Chem., 2008, 283(2): 919-928. |
| 42 | O'DONNELL M A, PEREZ-JIMENEZ E, OBERST A, et al.. Caspase 8 inhibits programmed necrosis by processing CYLD[J]. Nat. Cell Biol., 2011, 13(12): 1437-1442. |
| 43 | ZHU G, HERLYN M, YANG X. TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination[J]. Nat. Cell Biol., 2021, 23(9): 978-991. |
| 44 | XU S, TANG L, LI X, et al.. Immunotherapy for glioma: current management and future application[J]. Cancer Lett., 2020, 476: 1-12. |
| 45 | TUETTENBERG J, SEIZ M, MDEBATIN K, et al.. Pharmacokinetics, pharmacodynamics, safety and tolerability of APG101, a CD95-Fc fusion protein, in healthy volunteers and two glioma patients[J]. Int. Immunopharmacol., 2012, 13(1): 93-100. |
| 46 | WICK W, FRICKE H, JUNGE K, et al.. A phase Ⅱ, randomized, study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma[J]. Clin. Cancer Res. 2014, 20(24): 6304-6313. |
| 47 | WEI K C, HSU P W, TSAI H C, et al.. Safety and tolerability of asunercept plus standard radiotherapy/temozolomide in Asian patients with newly-diagnosed glioblastoma: a phase I study[J/OL]. Sci. Rep., 2021, 11(1): 24067[2024-04-07]. . |
| 48 | BLAES J, THOMÉ C M, NPFENNING P, et al.. Inhibition of CD95/CD95L (FAS/FASLG) signaling with APG101 prevents invasion and enhances radiation therapy for glioblastoma[J]. Mol. Cancer Res., 2018, 16(5): 767-776. |
| [1] | 布尔兰·叶尔肯别克, 郭文佳, 董晓刚. 骨膜蛋白在肿瘤微环境中的作用研究进展[J]. 生物技术进展, 2024, 14(2): 205-210. |
| [2] | 赵维坚, 徐弘庭, 肖向茜, 盛望. 肿瘤干细胞中的Hippo信号通路研究进展[J]. 生物技术进展, 2024, 14(2): 211-220. |
| [3] | 孙莉莉, 安外尔·约麦尔阿卜拉, 刘富中, 布尔兰·叶尔肯别克, 迪丽娜尔·叶尔夏提, 郭文佳. 基于肿瘤相关成纤维细胞基因构建乳腺癌预后预测模型及免疫浸润分析[J]. 生物技术进展, 2024, 14(2): 312-322. |
| [4] | 张鹏晓, 胡念. 黑色素瘤免疫治疗作用机制研究进展[J]. 生物技术进展, 2023, 13(6): 900-906. |
| [5] | 安外尔·约麦尔阿卜拉, 孙莉莉, 布尔兰·叶尔肯别克, 郭文佳. Piezo1在癌症中的作用研究进展[J]. 生物技术进展, 2023, 13(5): 712-717. |
| [6] | 马文博, 潘逸群, 王群, 马壮, 王明连, 杨怡姝. 烯壳氮化铁纳米磁珠用于捕获肺癌循环肿瘤细胞的初步研究[J]. 生物技术进展, 2023, 13(4): 628-636. |
| [7] | 李永超, 杨昭. 双特异抗体药物研发现状及发展对策[J]. 生物技术进展, 2023, 13(3): 353-358. |
| [8] | 高朗, 于思雪, 袁春森, 单志伟, 赵鹏翔. 黏蛋白在肿瘤免疫治疗中的研究进展[J]. 生物技术进展, 2023, 13(3): 390-398. |
| [9] | 唐颖, 武建强. 肉苁蓉苯乙醇苷的抗肿瘤作用[J]. 生物技术进展, 2023, 13(3): 399-405. |
| [10] | 董立强, 王斌, 苏适, 刘东琦. 雷公藤红素抗肿瘤作用及机制研究进展[J]. 生物技术进展, 2023, 13(1): 77-82. |
| [11] | 陈小巧, 牧仁, 李玉玲. 脐带间充质干细胞的生物学特性及旁分泌作用的研究进展[J]. 生物技术进展, 2022, 12(4): 559-567. |
| [12] | 王彩虹, 陈剑飞, 鞠久君, 朱雪洁, 王彩霞. PKM2促进肿瘤发展的作用机制研究[J]. 生物技术进展, 2022, 12(3): 411-418. |
| [13] | 武丽娜, 彭小忠, 鲁重美. 长链非编码RNA ZFAS1在结直肠癌中的表达及生物学功能[J]. 生物技术进展, 2022, 12(3): 427-435. |
| [14] | 王林琳, 孙振亮. 氨基酸转运体在肿瘤代谢中的研究进展[J]. 生物技术进展, 2022, 12(1): 50-56. |
| [15] | 李征, 郑亚民, 张小农. 镁离子与肿瘤的相关关系[J]. 生物技术进展, 2022, 12(1): 63-67. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部
