


生物技术进展 ›› 2024, Vol. 14 ›› Issue (2): 211-220.DOI: 10.19586/j.2095-2341.2023.0149
• 进展评述 • 上一篇
收稿日期:2023-11-21
接受日期:2024-01-02
出版日期:2024-03-25
发布日期:2024-04-17
通讯作者:
肖向茜,盛望
作者简介:赵维坚 E-mail: zhaowj@emails.bjut.edu.cn
基金资助:
Weijian ZHAO( ), Hongting XU, Xiangqian XIAO(
), Hongting XU, Xiangqian XIAO( ), Wang SHENG(
), Wang SHENG( )
)
Received:2023-11-21
Accepted:2024-01-02
Online:2024-03-25
Published:2024-04-17
Contact:
Xiangqian XIAO,Wang SHENG
摘要:
如何抑制肿瘤细胞的增殖和干性基因的表达已经成为近年来科学家在癌症治疗领域关注的重点之一。肿瘤干细胞(cancer stem cell,CSC)是肿瘤中一类具有自我更新能力的细胞类群,被认为是肿瘤发生过程中的驱动因素之一。Hippo信号通路在生物进化过程中高度保守,且对细胞增殖、组织发育和器官大小具有重要作用。简述了Hippo信号通路及其在肿瘤干细胞中的作用机制,并对肿瘤的治疗提出了展望,以期为肿瘤干细胞的发展和未来治疗提供参考。
中图分类号:
赵维坚, 徐弘庭, 肖向茜, 盛望. 肿瘤干细胞中的Hippo信号通路研究进展[J]. 生物技术进展, 2024, 14(2): 211-220.
Weijian ZHAO, Hongting XU, Xiangqian XIAO, Wang SHENG. Research Progress on Hippo Signaling Pathway in Cancer Stem Cell[J]. Current Biotechnology, 2024, 14(2): 211-220.
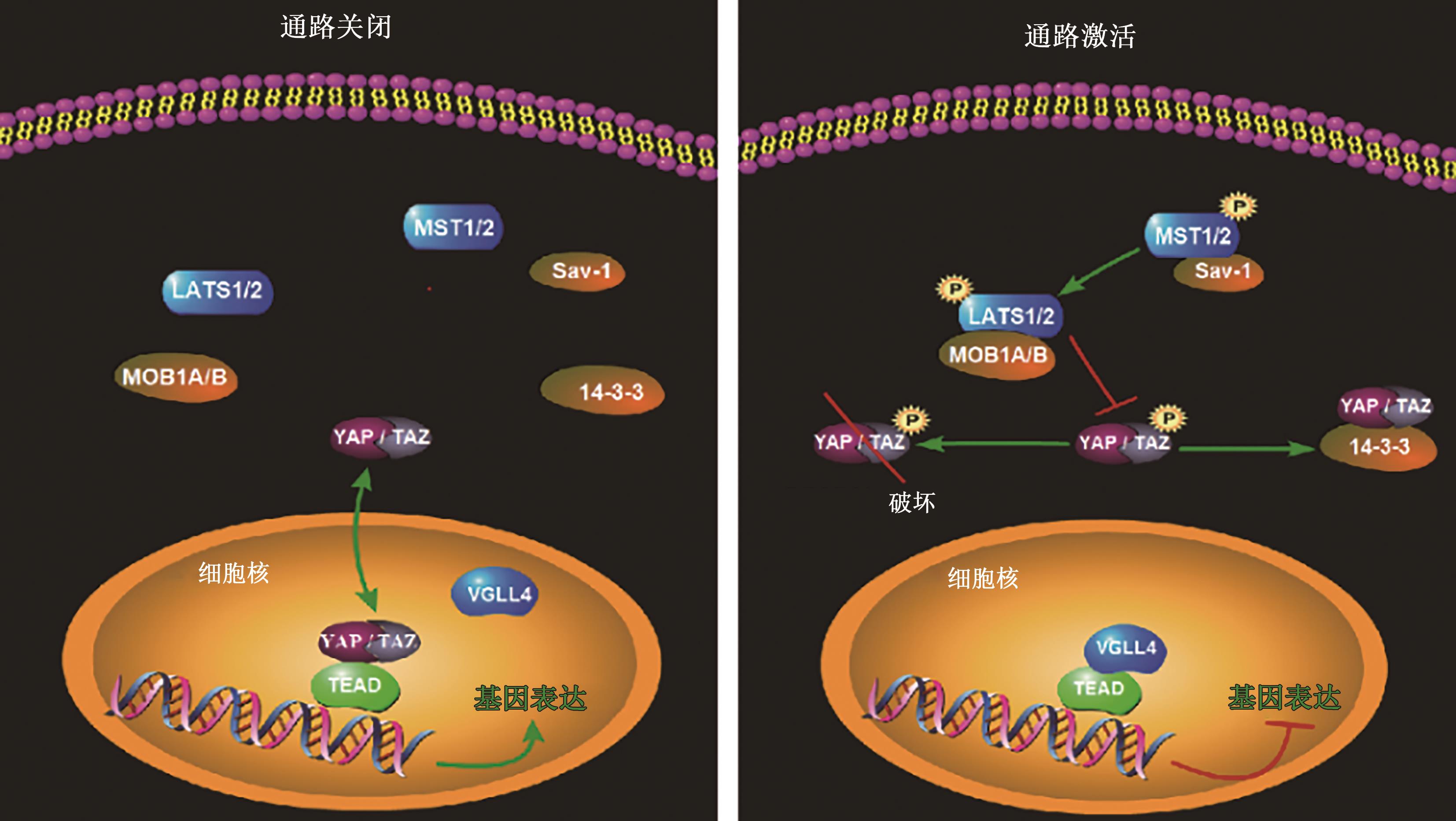
图1 Hippo信号通路的关闭与开启状态注:MST1/2—STE20样激酶1/2;LATS1/2—大型肿瘤抑制激酶1/2;Sav-1—萨尔瓦多蛋白-1;MOB1A/B—MOB激活蛋白-1;YAP—Yes相关蛋白;TAZ—具有PDZ结合结构域的转录激活剂;TEAD—转录增强缔合域蛋白;14-3-3—14-3-3蛋白;VGLL4—Vestigial样家族蛋白4。
Fig. 1 The closed and open states of the Hippo signal pathway
| 压力形式 | 对Hippo通路的影响 | 参考文献 |
|---|---|---|
| 缺氧应激 | 降低YAP磷酸化水平,促进YAP核定位 | [ |
| 氧化应激 | 促进YAP核定位 | [ |
| 热应激 | 诱导YAP去磷酸化和活化 | [ |
| 缺血性损伤 | 增加YAP的表达对组织缺血再灌注保护的反应 | [ |
| 高渗胁迫 | 诱导TAZ的酪氨酸磷酸化,TEAD细胞质定位 | [ |
表1 不同应激方式对Hippo信号通路的影响
Table 1 Effects of different stress methods on Hippo signal pathway
| 压力形式 | 对Hippo通路的影响 | 参考文献 |
|---|---|---|
| 缺氧应激 | 降低YAP磷酸化水平,促进YAP核定位 | [ |
| 氧化应激 | 促进YAP核定位 | [ |
| 热应激 | 诱导YAP去磷酸化和活化 | [ |
| 缺血性损伤 | 增加YAP的表达对组织缺血再灌注保护的反应 | [ |
| 高渗胁迫 | 诱导TAZ的酪氨酸磷酸化,TEAD细胞质定位 | [ |
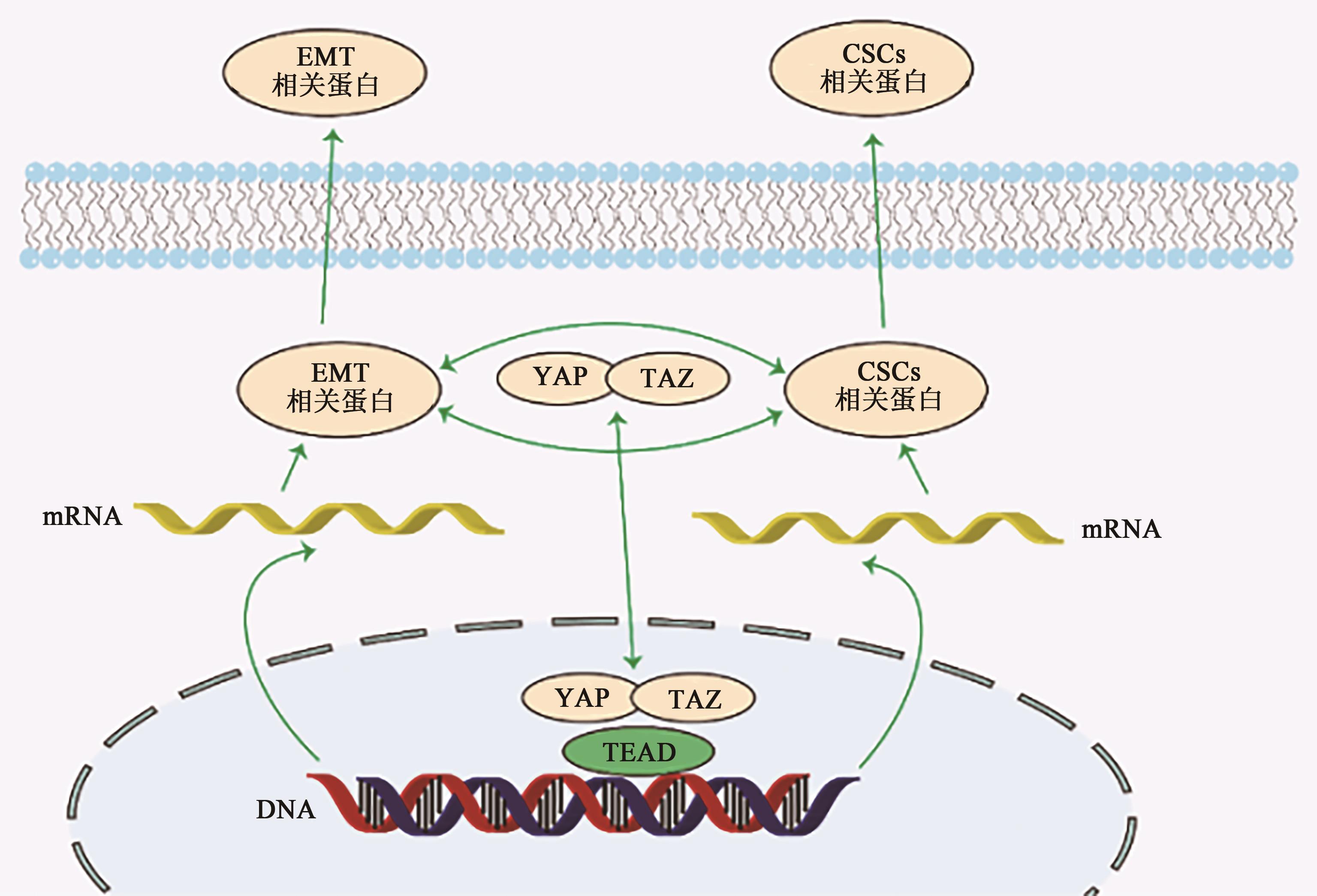
图2 Hippo信号通路介导CSCs与EMT之间的串联注:EMT相关蛋白—上皮-间充质转化相关蛋白;CSCs相关蛋白—肿瘤干细胞相关蛋白;YAP—Yes相关蛋白;TAZ—具有PDZ结合结构域的转录激活剂;TEAD—转录增强缔合域蛋白。
Fig. 2 Hippo signaling pathway mediates the cascade between CSCs and EMT
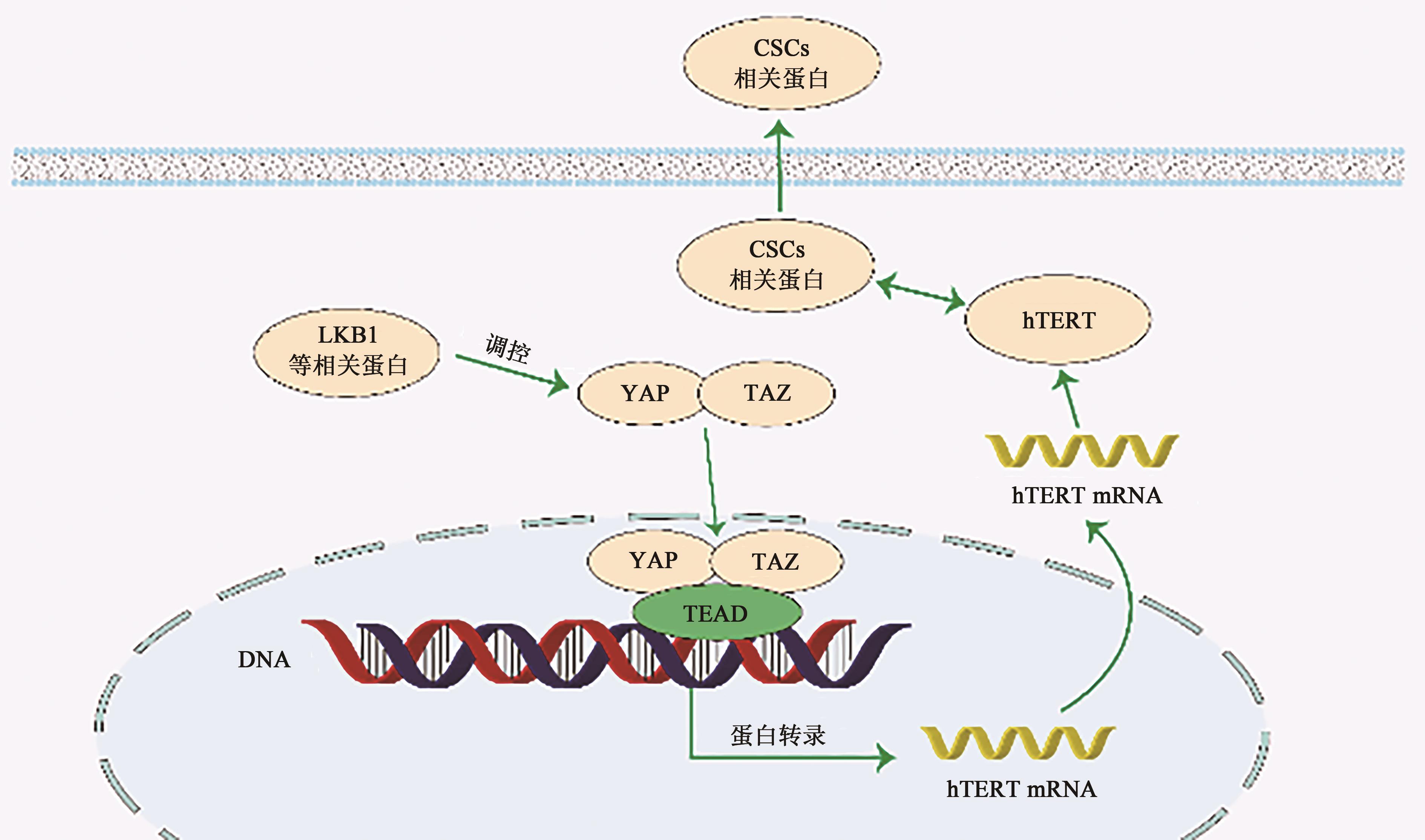
图3 Hippo信号通路与hTERT和CSCs的关系注:YAP—Yes相关蛋白;TAZ—具有PDZ结合结构域的转录激活剂;TEAD—转录增强缔合域蛋白;hTERT—人端粒酶逆转录酶; CSCs 相关蛋白—肿瘤干细胞相关蛋白;LKB1—肝脏激酶家族B1。
Fig. 3 The relationship between Hippo signal pathway and hTERT and CSCs
| 1 | SINGH D, VIGNAT J, LORENZONI V, et al.. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative[J]. Lancet Glob. Health, 2023, 11(2): 197-206. |
| 2 | VLASHI E, PAJONK F. Cancer stem cells, cancer cell plasticity and radiation therapy[J]. Semin. Cancer Biol., 2015, 31: 28-35. |
| 3 | PAN D. The hippo signaling pathway in development and cancer[J]. Dev. Cell, 2010, 19(4): 491-505. |
| 4 | MOHAJAN S, JAISWAL P K, VATANMAKARIAN M, et al.. Hippo pathway: regulation, deregulation and potential therapeutic targets in cancer[J]. Cancer Lett., 2021, 507: 112-123. |
| 5 | TANG W, LI M, YANGZHONG X, et al.. Hippo signaling pathway and respiratory diseases[J/OL]. Cell Death Discov., 2022, 8(1): 213[2024-02-02]. . |
| 6 | WU Z, GUAN K L. Hippo signaling in embryogenesis and development[J]. Trends Biochem. Sci., 2021, 46(1): 51-63. |
| 7 | CHEN R, XIE R, MENG Z, et al.. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade[J]. Nat. Cell Biol., 2019, 21(12): 1565-1577. |
| 8 | PRASKOVA M, XIA F, AVRUCH J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation[J]. Curr. Biol., 2008, 18(5): 311-321. |
| 9 | HERGOVICH A, SCHMITZ D, HEMMINGS B A. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane[J]. Biochem. Biophys. Res. Commun., 2006, 345(1): 50-58. |
| 10 | ZHAO B, LI L, TUMANENG K, et al.. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP)[J]. Genes Dev., 2010, 24(1): 72-85. |
| 11 | HILGER D, MASUREEL M, KOBILKA B K. Structure and dynamics of GPCR signaling complexes[J]. Nat. Struct. Mol. Biol., 2018, 25(1): 4-12. |
| 12 | YU F X, ZHANG Y, PARK H W, et al.. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation[J]. Genes Dev., 2013, 27(11): 1223-1232. |
| 13 | KIM M, KIM M, LEE S, et al.. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes[J]. EMBO J., 2013, 32(11): 1543-1555. |
| 14 | CHO-CHUNG Y S. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy[J]. Cancer Res., 1990, 50(22): 7093-7100. |
| 15 | MILLER E, YANG J, DERAN M, et al.. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP[J]. Chem. Biol., 2012, 19(8): 955-962. |
| 16 | REGUÉ L, MOU F, AVRUCH J. G protein-coupled receptors engage the mammalian Hippo pathway through F-actin: F-Actin, assembled in response to Galpha12/13 induced RhoA-GTP, promotes dephosphorylation and activation of the YAP oncogene[J]. BioEssays Issues Rev. Mol. Cell. Dev. Biol., 2013, 35(5): 430-435. |
| 17 | HE H, SUGIYAMA A, SNYDER N W, et al.. Acyl-coA thioesterase 12 suppresses YAP-mediated hepatocarcinogenesis by limiting glycerolipid biosynthesis[J/OL]. Cancer Lett., 2023, 565: 216210[2024-02-02]. . |
| 18 | WANG Z, LIU P, ZHOU X, et al.. Endothelin promotes colorectal tumorigenesis by activating YAP/TAZ[J]. Cancer Res., 2017, 77(9): 2413-2423. |
| 19 | ZHOU X, WANG S, WANG Z, et al.. Estrogen regulates Hippo signaling via GPER in breast cancer[J]. J. Clin. Investig., 2015, 125(5): 2123-2135. |
| 20 | LI H, LI Q, DANG K, et al.. YAP/TAZ activation drives uveal melanoma initiation and progression[J]. Cell Rep., 2019, 29(10): 3200-3211. |
| 21 | SU S, JIANG W, WANG X, et al.. Resolvin D1 inhibits the proliferation of osteoarthritis fibroblast-like synoviocytes through the Hippo-YAP signaling pathway[J/OL]. BMC Musculoskelet. Disord., 2022, 23(1): 149[2024-02-02]. . |
| 22 | ZHANG K, HU Z, QI H, et al.. G-protein-coupled receptors mediate ω-3 PUFAs-inhibited colorectal cancer by activating the Hippo pathway[J]. Oncotarget, 2016, 7(36): 58315-58330. |
| 23 | AZZOLIN L, ZANCONATO F, BRESOLIN S, et al.. Role of TAZ as mediator of Wnt signaling[J]. Cell, 2012, 151(7): 1443-1456. |
| 24 | AZZOLIN L, PANCIERA T, SOLIGO S, et al.. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response[J]. Cell, 2014, 158(1): 157-170. |
| 25 | JIA X, WU B, HUANG J, et al.. YAP and Wnt3a independently promote AECIIs proliferation and differentiation by increasing nuclear β-cateninexpression in experimental bronchopulmonary dysplasia[J]. Int. J. Mol. Med., 2021, 47(1): 195-206. |
| 26 | MALGUNDKAR S H, BURNEY I, MOUNDHRI M A, et al.. E2F5 promotes the malignancy of ovarian cancer via the regulation of hippo and Wnt pathways[J]. Genet. Test. Mol. Biomark., 2021, 25(3): 179-186. |
| 27 | MALGUNDKAR S H, BURNEY I, MOUNDHRI M A, et al.. FAT4 silencing promotes epithelial-to-mesenchymal transition and invasion via regulation of YAP and β-catenin activity in ovarian cancer[J/OL]. BMC Cancer, 2020, 20(1): 374[2024-02-02]. . |
| 28 | KIM W, KHAN S K, GVOZDENOVIC-JEREMIC J, et al.. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis[J]. J. Clin. Investig., 2017, 127(1): 137-152. |
| 29 | ZHAO B, WEI X, LI W, et al.. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control[J]. Genes Dev., 2007, 21(21): 2747-2761. |
| 30 | ZHAO B, LI L, WANG L, et al.. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis[J]. Genes Dev., 2012, 26(1): 54-68. |
| 31 | WAN W, MIAO Y, NIU Y, et al.. Human umbilical cord mesenchymal stem cells conditioned medium exerts anti-tumor effects on KGN cells in a cell density-dependentmanner through activation of the Hippo pathway[J/OL]. Stem Cell Res. Ther., 2023, 14(1): 46[2024-02-02]. . |
| 32 | KUMAR B, AHMAD R, GIANNICO G A, et al.. Claudin-2 inhibits renal clear cell carcinoma progression by inhibiting YAP-activation[J/OL]. J. Exp. Clin. Cancer Res., 2021, 40(1): 77[2024-02-02]. . |
| 33 | PAQUET-FIFIELD S, KOH S L, CHENG L, et al.. Tight junction protein claudin-2 promotes self-renewal of human colorectal cancer stem-like cells[J]. Cancer Res., 2018, 78(11): 2925-2938. |
| 34 | YU S, ZHANG Y, LI Q, et al.. CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer[J/OL]. Cell Death Dis., 2019, 10(12): 949[2024-02-02]. . |
| 35 | KONG F E, LI G M, TANG Y Q, et al.. Targeting tumor lineage plasticity in hepatocellular carcinoma using an anti-CLDN6 antibody-drug conjugate[J/OL]. Sci. Transl. Med., 2021, 13(579): eabb6282[2024-02-02]. . |
| 36 | KOTTON D N. Claudin-18: unexpected regulator of lung alveolar epithelial cell proliferation[J]. J. Clin. Investig., 2018, 128(3): 903-905. |
| 37 | ZHOU B, FLODBY P, LUO J, et al.. Claudin-18-mediated YAP activity regulates lung stem and progenitor cell homeostasis and tumorigenesis[J]. J. Clin. Investig., 2018, 128(3): 970-984. |
| 38 | XU X, LI Y, ZHANG R, et al.. Jianpi Yangzheng decoction suppresses gastric cancer progression via modulating the miR-448/CLDN18.2 mediated YAP/TAZ signaling[J/OL]. J. Ethnopharmacol., 2023, 311: 116450[2024-02-02]. . |
| 39 | DUPONT S, MORSUT L, ARAGONA M, et al.. Role of YAP/TAZ in mechanotransduction[J]. Nature, 2011, 474(7350): 179-183. |
| 40 | PATWARDHAN S, MAHADIK P, SHETTY O, et al.. ECM stiffness-tuned exosomes drive breast cancer motility through thrombospondin-1[J/OL]. Biomaterials, 2021, 279: 121185[2024-02-02]. . |
| 41 | MAHADIK P, PATWARDHAN S. ECM stiffness-regulated exosomal thrombospondin-1 promotes tunneling nanotubes-based cellular networking in breast cancer cells[J/OL]. Arch. Biochem. Biophys., 2023, 742: 109624[2024-02-02]. . |
| 42 | DENG B, ZHAO Z, KONG W, et al.. Biological role of matrix stiffness in tumor growth and treatment[J/OL]. J. Transl. Med., 2022, 20(1): 540[2024-02-02]. . |
| 43 | LIU Z, HAYASHI H, MATSUMURA K, et al.. Hyperglycaemia induces metabolic reprogramming into a glycolytic phenotype and promotes epithelial-mesenchymal transitions via YAP/TAZ-Hedgehog signalling axis in pancreatic cancer[J]. Br. J. Cancer, 2023, 128(5): 844-856. |
| 44 | MO J S, MENG Z, KIM Y C, et al.. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway[J]. Nat. Cell Biol., 2015, 17(4): 500-510. |
| 45 | LI X, FAN S, CAI C, et al.. YAP regulates the liver size during the fasting-refeeding transition in mice[J]. Acta Pharm. Sin. B, 2023, 13(4): 1588-1599. |
| 46 | LUO M, MENG Z, MOROISHI T, et al.. Heat stress activates YAP/TAZ to induce the heat shock transcriptome[J]. Nat. Cell Biol., 2020, 22(12): 1447-1459. |
| 47 | LIU Y, LU T, ZHANG C, et al.. Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury[J]. J. Hepatol., 2019, 71(4): 719-730. |
| 48 | ZHANG X, LI Y, MA Y, et al.. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress[J/OL]. J. Exp. Clin. Cancer Res., 2018, 37(1): 216[2024-02-02]. . |
| 49 | HUANG Y, JEDLIČKOVÁ H, CAI Y, et al.. Oxidative stress-mediated YAP dysregulation contributes to the pathogenesis of Pemphigus vulgaris [J/OL]. Front. Immunol., 2021, 12: 649502[2024-02-02]. . |
| 50 | SONG H, QIU Z, WANG Y, et al.. HIF-1α/YAP signaling rewrites glucose/iodine metabolism program to promote papillary thyroid cancer progression[J]. Int. J. Biol. Sci., 2023, 19(1): 225-241. |
| 51 | YU H, WANG H, LIU J, et al.. The effect of ROS-YAP crosstalk on osteoimmune response orchestrating osteogenesis[J]. Cell Cycle Georget. Tex, 2023, 22(11): 1391-1405. |
| 52 | MASUDA H, ARISAKA Y, HAKARIYA M, et al.. Molecular mobility of polyrotaxane surfaces alleviates oxidative stress-induced senescence in mesenchymal stem cells[J/OL]. Macromol. Biosci., 2023, 23(5): e2300053[2024-02-02]. . |
| 53 | JIN J, ZHANG L, LI X, et al.. Oxidative stress-CBP axis modulates MOB1 acetylation and activates the Hippo signaling pathway[J]. Nucleic Acids Res., 2022, 50(7): 3817-3834. |
| 54 | JIANG X, MARUYAMA J, IWASA H, et al.. Heat shock induces the nuclear accumulation of YAP1 via SRC[J/OL]. Exp. Cell Res., 2021, 399(1): 112439[2024-02-02]. . |
| 55 | CHEN X, TONG G, CHEN S. Basic fibroblast growth factor protects against liver ischemia-reperfusion injury via the Nrf2/Hippo signaling pathway[J/OL]. Tissue Cell, 2022, 79: 101921[2024-02-02]. . |
| 56 | JANG E J, JEONG H, HAN K H, et al.. TAZ suppresses NFAT5 activity through tyrosine phosphorylation[J]. Mol. Cell. Biol., 2012, 32(24): 4925-4932. |
| 57 | LIN K C, MOROISHI T, MENG Z, et al.. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation[J]. Nat. Cell Biol., 2017, 19(8): 996-1002. |
| 58 | ALHOUSAMI T, DINY M, ALI F, et al.. Inhibition of LSD1 attenuates oral cancer development and promotes therapeutic efficacy of immune checkpoint blockade and YAP/TAZ inhibition[J]. Mol. Cancer Res., 2022, 20(5): 712-721. |
| 59 | DELVAUX M, HAGUÉ P, CRACIUN L, et al.. Ferroptosis induction and YAP inhibition as new therapeutic targets in gastrointestinal stromal tumors (GISTs)[J/OL]. Cancers, 2022, 14(20): 5050[2024-02-02]. . |
| 60 | PHILIPPE C, PINSON B, DOMPIERRE J, et al.. AICAR antiproliferative properties involve the AMPK-independent activation of the tumor suppressors LATS 1 and 2[J]. Neoplasia, 2018, 20(6): 555-562. |
| 61 | TANG T T, KONRADI A W, FENG Y, et al.. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma[J]. Mol. Cancer Ther., 2021, 20(6): 986-998. |
| 62 | SAITO Y, YIN D, KUBOTA N, et al.. A therapeutically targetable TAZ-TEAD2 pathway drives the growth of hepatocellular carcinoma via ANLN and KIF23[J]. Gastroenterology, 2023, 164(7): 1279-1292. |
| 63 | HEINRICH T, PETERSON C, SCHNEIDER R, et al.. Optimization of TEAD P-site binding fragment hit into in vivo active lead MSC-4106[J]. J. Med. Chem., 2022, 65(13): 9206-9229. |
| 64 | LAPIDOT T, SIRARD C, VORMOOR J, et al.. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice[J]. Nature, 1994, 367(6464): 645-648. |
| 65 | ZHANG H, BROWN R L, WEI Y, et al.. CD44 splice isoform switching determines breast cancer stem cell state[J]. Genes Dev., 2019, 33(3-4): 166-179. |
| 66 | JIANG P, LI F, LIU Z, et al.. BTB and CNC homology 1 (Bach1) induces lung cancer stem cell phenotypes by stimulating CD44 expression[J/OL]. Respir. Res., 2021, 22(1): 320[2024-02-02]. . |
| 67 | KOYAMA S, TSUCHIYA H, AMISAKI M, et al.. NEAT1 is required for the expression of the liver cancer stem cell marker CD44[J/OL]. Int. J. Mol. Sci., 2020, 21(6): 1927[2024-02-02]. . |
| 68 | LI H, WANG C, LAN L, et al.. METTL3 promotes oxaliplatin resistance of gastric cancer CD133+ stem cells by promoting PARP1 mRNA stability[J/OL]. Cell. Mol. Life Sci., 2022, 79(3): 135[2024-02-02]. . |
| 69 | THIRUSANGU P, RAY U, SARKAR B S, et al.. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma[J]. Oncogene, 2022, 41(33): 4003-4017. |
| 70 | YANG J, ALJITAWI O, VAN VELDHUIZEN P. Prostate cancer stem cells: the role of CD133[J/OL]. Cancers, 2022, 14(21): 5448[2024-02-02]. . |
| 71 | FERRAGUT F, VACHETTA V S, TRONCOSO M F, et al.. ALCAM/CD166: a pleiotropic mediator of cell adhesion, stemness and cancer progression[J]. Cytokine Growth Factor Rev., 2021, 61: 27-37. |
| 72 | CHEN X, LIANG R, LIN H, et al.. CD166 promotes cancer stem cell-like phenotype via the EGFR/ERK1/2 pathway in the nasopharyngeal carcinoma cell line CNE-2R[J/OL]. Life Sci., 2021, 267: 118983[2024-02-02]. . |
| 73 | LEVIN T G, POWELL A E, DAVIES P S, et al.. Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract[J]. Gastroenterology, 2010, 139(6): 2072-2082.e5. |
| 74 | PRASETYANTI P R, MEDEMA J P. Intra-tumor heterogeneity from a cancer stem cell perspective[J/OL]. Mol. Cancer, 2017, 16(1): 41[2024-02-02]. . |
| 75 | PASTUSHENKO I, BLANPAIN C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol., 2019, 29(3): 212-226. |
| 76 | QIN T, LI B, FENG X, et al.. Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity[J/OL]. J. Exp. Clin. Cancer Res., 2018, 37(1): 287[2024-02-02]. . |
| 77 | PASTUSHENKO I, MAURI F, SONG Y, et al.. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis[J]. Nature, 2021, 589(7842): 448-455. |
| 78 | FELDKER N, FERRAZZI F, SCHUHWERK H, et al.. Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer[J/OL]. EMBO J., 2020, 39(17): e103209[2024-02-02]. . |
| 79 | PARK H, LEE Y, LEE K, et al.. The clinicopathological significance of YAP/TAZ expression in hepatocellular carcinoma with relation to hypoxia and stemness[J/OL]. Pathol. Oncol. Res., 2021, 27: 604600[2024-02-02]. . |
| 80 | SANTORO R, ZANOTTO M, CARBONE C, et al.. MEKK3 sustains EMT and stemness in pancreatic cancer by regulating YAP and TAZ transcriptional activity[J]. Anticancer Res., 2018, 38(4): 1937-1946. |
| 81 | LIU M, ZHANG Y, YANG J, et al.. Zinc-dependent regulation of ZEB1 and YAP1 coactivation promotes epithelial-mesenchymal transition plasticity and metastasis in pancreatic cancer[J]. Gastroenterology, 2021, 160(5): 1771-1783. |
| 82 | WANG Y, LIAO R, CHEN X, et al.. Twist-mediated PAR1 induction is required for breast cancer progression and metastasis by inhibiting Hippo pathway[J/OL]. Cell Death Dis., 2020, 11(7): 520[2024-02-02]. . |
| 83 | LIU Y, SONG Y, CAO M, et al.. A novel EHD1/CD44/Hippo/SP1 positive feedback loop potentiates stemness and metastasis in lung adenocarcinoma[J/OL]. Clin. Transl. Med., 2022, 12(4): e836[2024-02-02]. . |
| 84 | KAOWINN S, YAWUT N, KOH S S, et al.. Cancer upregulated gene CUG2 elevates YAP1 expression, leading to enhancement of epithelial-mesenchymal transition in human lung cancer cells[J]. Biochem. Biophys. Res. Commun., 2019, 511(1): 122-128. |
| 85 | LIN H, PENG J, ZHU T, et al.. Exosomal miR-4800-3p aggravates the progression of hepatocellular carcinoma via regulating the hippo signaling pathway by targeting STK25[J/OL]. Front. Oncol., 2022, 12: 759864[2024-02-02]. . |
| 86 | LIU J, HONG X, LIANG C Y, et al.. Simultaneous visualisation of the complete sets of telomeres from the Mme I generated terminal restriction fragments in yeasts[J]. Yeast Chichester Engl., 2020, 37(11): 585-595. |
| 87 | WANG H, GONG P, CHEN T, et al.. Colorectal cancer stem cell states uncovered by simultaneous single-cell analysis of transcriptome and telomeres[J/OL]. Adv. Sci., 2021, 8(8): 2004320[2024-02-02]. . |
| 88 | PIÑOL-FELIS C, FERNÁNDEZ-MARCELO T, VIÑAS-SALAS J, et al.. Telomeres and telomerase in the clinical management of colorectal cancer[J]. Clin. Transl. Oncol., 2017, 19(4): 399-408. |
| 89 | LIU Z, LI Q, LI K, et al.. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells[J]. Oncogene, 2013, 32(36): 4203-4213. |
| 90 | ZHANG Q, LIU N, BAI J, et al.. Human telomerase reverse transcriptase is a novel target of Hippo-YAP pathway[J]. FASEB J., 2020, 34(3): 4178-4188. |
| 91 | HE L, WU M Z, WANG X B, et al.. Tumor suppressor LKB1 inhibits both the mRNA expression and the amplification of hTERC by the phosphorylation of YAP in lung cancer cells[J]. J. Cancer, 2019, 10(16): 3632-3638. |
| 92 | YU M, PENG Z, QIN M, et al.. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation[J]. Mol. Cell, 2021, 81(6): 1216-1230. |
| [1] | 张鹏晓, 胡念. 黑色素瘤免疫治疗作用机制研究进展[J]. 生物技术进展, 2023, 13(6): 900-906. |
| [2] | 杜琳琳, 谢飞, 马雪梅. SALL4的促癌功能及治疗意义[J]. 生物技术进展, 2023, 13(5): 704-711. |
| [3] | 王林琳, 孙振亮. 氨基酸转运体在肿瘤代谢中的研究进展[J]. 生物技术进展, 2022, 12(1): 50-56. |
| [4] | 肖金平, 李程, 曹云娣, 孙志坚, 康平, 兰晓梅. RET原癌基因与肿瘤相关性研究的进展现状[J]. 生物技术进展, 2022, 12(1): 57-62. |
| [5] | 刘程林子,吴耀钦,黄恩泽,徐瑞丰,郑鹏,. 双硫仑抗癌作用研究进展[J]. 生物技术进展, 2021, 11(2): 155-162. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部