


生物技术进展 ›› 2024, Vol. 14 ›› Issue (5): 697-711.DOI: 10.19586/j.2095-2341.2024.0104
• 进展评述 •
吴焕振1( ), 杨野1,2,3,4, 崔秀明1,2,3,4, 刘源1,2,3,4(
), 杨野1,2,3,4, 崔秀明1,2,3,4, 刘源1,2,3,4( )
)
收稿日期:2024-05-22
接受日期:2024-08-01
出版日期:2024-09-25
发布日期:2024-10-22
通讯作者:
刘源
作者简介:吴焕振 E-mail:whz1632024@163.com;
基金资助:
Huanzhen WU1( ), Ye YANG1,2,3,4, Xiuming CUI1,2,3,4, Yuan LIU1,2,3,4(
), Ye YANG1,2,3,4, Xiuming CUI1,2,3,4, Yuan LIU1,2,3,4( )
)
Received:2024-05-22
Accepted:2024-08-01
Online:2024-09-25
Published:2024-10-22
Contact:
Yuan LIU
摘要:
在全球人口增长和耕地减少的双重压力下,农业的可持续发展迫在眉睫。生物防治通过利用天敌、微生物等有益生物抑制害虫和病原体,展现出巨大的潜力,是现代农业病虫害防治的有效途径。概述了生物防治在农业可持续发展中的重要性及其在保护生物多样性和环境中的积极作用,详述了害虫天敌的应用、有益微生物防治植物病害、拮抗菌筛选技术的发展,以及组学技术和纳米技术的应用。最后,提出若干改进策略,旨在为生物防治相关研究和实际应用提供有价值的参考和指导,从而提高对生物防治技术的认识和应用,促进农业可持续发展。
中图分类号:
吴焕振, 杨野, 崔秀明, 刘源. 农业生物防治技术的现状及改进策略[J]. 生物技术进展, 2024, 14(5): 697-711.
Huanzhen WU, Ye YANG, Xiuming CUI, Yuan LIU. The Current Status and Improvement Strategies of Agricultural Biological Control Technology[J]. Current Biotechnology, 2024, 14(5): 697-711.
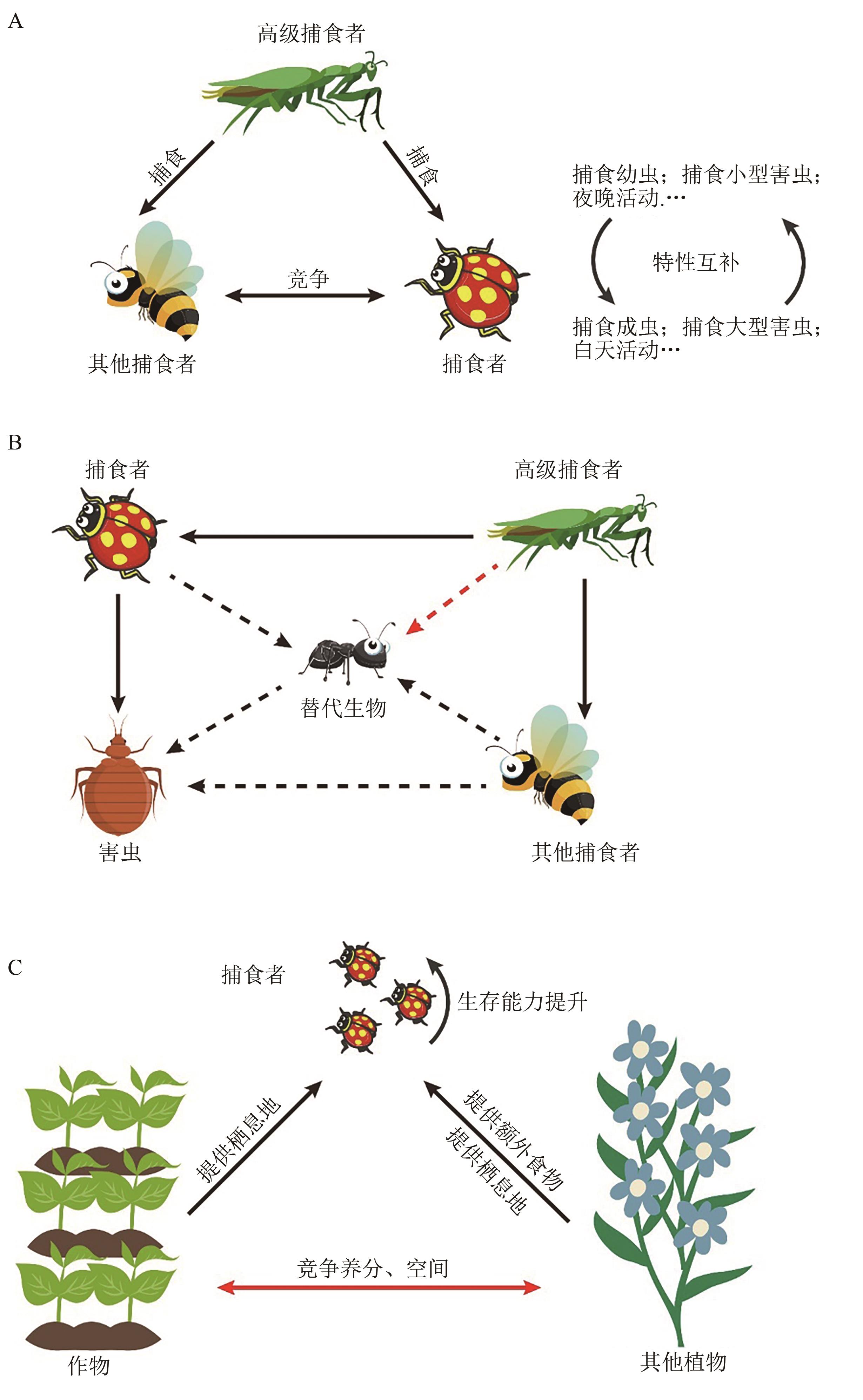
图1 天敌、替代生物和非作物植物对生物防治和农业生产的影响A:多样性的天敌在特征互补的情况下能发挥更好的生物防治效果,而天敌之间的捕食和竞争会降低平均捕食率;B:替代猎物/宿主直接作用于害虫天敌(虚线)且为次要目标时可以加快天敌的繁殖,提高其捕食害虫的能力,在作为主要目标的情况下会导致其对害虫的捕食率下降,若替代生物被高级捕食者优先捕食(红色虚线),也可以起到保护害虫天敌的作用;C:非作物植物可以提供额外食物和栖息地提高天敌的种群数量和生存能力,而与作物竞争养分和生存空间的植物会导致作物产量的降低。部分素材来源于www.freepik.com。
Fig. 1 The impact of natural enemies, alternative organisms, and non-crop plants on biological control and agricultural production
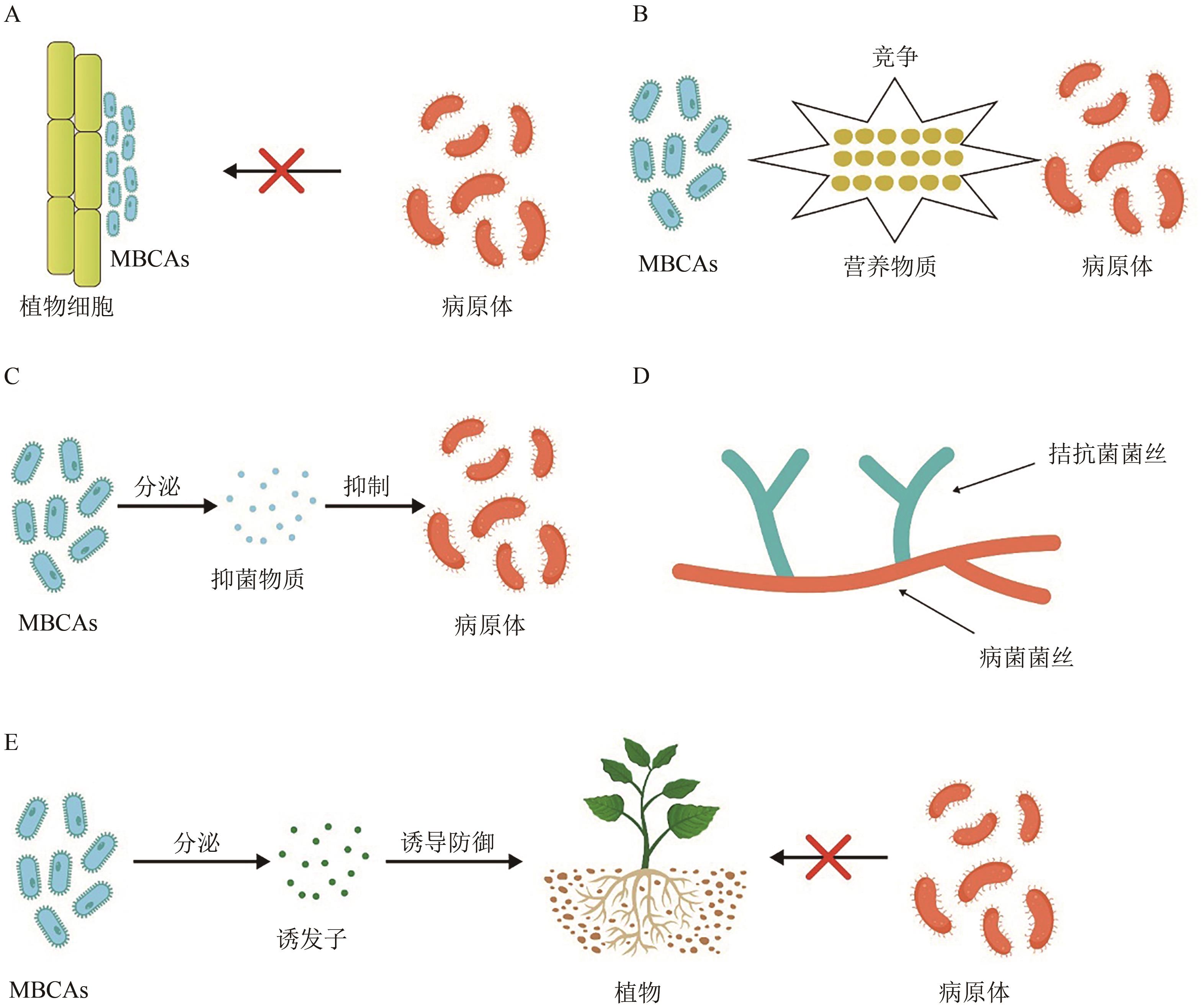
图3 MBCA发挥生物防治作用的方式A:微生物防治剂形成生物膜阻碍病原菌对植物的感染;B:微生物防治剂与病原菌争夺养分;C:微生物防治剂分泌抗菌化合物抑制病原菌;D:微生物拮抗菌的菌丝寄生在病原菌菌丝上汲取其养分,减缓病原菌的生长繁殖;E:微生物拮抗菌产生诱发因子通过ISR途径使植物获得对病原菌的抗性
Fig. 3 The ways in which MBCA exert biological control effects
| 1 | BERNARDES M F F, PAZIN M, PEREIRA L C, et al.. Impact of pesticides on environmental and human health[M]// Toxicology Studies-Cells, Drugs and Environment, 2015. |
| 2 | RANI L, THAPA K, KANOJIA N, et al.. An extensive review on the consequences of chemical pesticides on human health and environment[J/OL]. J. Clean. Prod., 2021, 283: 124657[2024-07-28]. . |
| 3 | ZHAO J, LUO Q, DENG H, et al.. Opportunities and challenges of sustainable agricultural development in China[J]. Philos. Trans. R. Soc. Lond. B Biol. Sci., 2008, 363(1492): 893-904. |
| 4 | STENBERG J, SUNDH I, BECHER P, et al.. Correction to: When is it biological control? A framework of definitions, mechanisms, and classifications[J]. J. Pest Sci., 2021, 94: 677-677. |
| 5 | BALE J S, VAN LENTEREN J C, BIGLER F. Biological control and sustainable food production[J]. Philos. Trans. R. Soc. Lond. B Biol. Sci., 2008, 363(1492): 761-776. |
| 6 | SATHISH B, SINGH V V, KUMAR S, et al.. Incremental cost-benefit ratio of certain chemical and bio-pesticides against tomato fruit borer, Helicoverpa armigera Hubner (Noctuidae: Lepidoptera) in tomato crop[J]. Bull. Environ. Pharmacol. Life Sci., 2018, 7: 102-106. |
| 7 | AHMAD A S. Mechanisms of action and biocontrol potential of Trichoderma against fungal plant diseases-A review[J/OL]. Ecol. Complex., 2022, 49: 100978[2024-07-28]. . |
| 8 | SNYDER W E. Give predators a complement: conserving natural enemy biodiversity to improve biocontrol[J]. Biol. Contr., 2019, 135: 73-82. |
| 9 | LOSEY J E, DENNO R F. Positive predator-predator interactions: enhanced predation rates and synergistic suppression of aphid populations[J/OL]. Ecology, 1998, 79(6): 2143[2024-07-28]. . |
| 10 | JONSSON M, KAARTINEN R, STRAUB C S. Relationships between natural enemy diversity and biological control[J]. Curr. Opin. Insect Sci., 2017, 20: 1-6. |
| 11 | AIGBEDION-ATALOR P O, HILL M P, AYELO P M, et al.. Can the combined use of the mirid predator Nesidiocoris tenuis and a braconid larval endoparasitoid Dolichogenidea gelechiidivoris improve the biological control of Tuta absoluta?[J/OL]. Insects, 2021, 12(11): 1004[2024-07-28]. . |
| 12 | AZEVEDO L H, LEITE L G, CHACON-OROZCO J G, et al.. Free living nematodes as alternative prey for soil predatory mites: an interdisciplinary case study of conservation biological control[J]. Biol. Contr., 2019, 132: 128-134. |
| 13 | LETOURNEAU D K, ARMBRECHT I, RIVERA B S, et al.. Does plant diversity benefit agroecosystems? A synthetic review[J]. Ecol. Appl., 2011, 21(1): 9-21. |
| 14 | IRVIN N A, PIERCE C, HODDLE M S. Evaluating the potential of flowering plants for enhancing predatory hoverflies (Syrphidae) for biological control of Diaphorina citri (Liviidae) in California[J/OL]. Biol. Contr., 2021, 157: 104574[2024-07-28]. . |
| 15 | SNYDER W E, IVES A R. Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol[J]. Ecology, 2003, 84(1): 91-107. |
| 16 | LANDIS D A, WRATTEN S D, GURR G M. Habitat management to conserve natural enemies of arthropod pests in agriculture[J]. Annu. Rev. Entomol., 2000, 45: 175-201. |
| 17 | DUNNING J B, DANIELSON B J, PULLIAM H R. Ecological processes that affect populations in complex landscapes[J/OL]. Oikos, 1992, 65(1): 169[2024-07-28]. . |
| 18 | BERENDSEN R L, PIETERSE C M J, BAKKER P A H M. The rhizosphere microbiome and plant health[J]. Trends Plant Sci., 2012, 17(8): 478-486. |
| 19 | JIAO S, WANG J, WEI G, et al.. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances[J]. Chemosphere, 2019, 235: 248-259. |
| 20 | QI Y, LIU H, ZHANG B, et al.. Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng[J/OL]. Appl. Soil Ecol., 2022, 172: 104369[2024-07-28]. . |
| 21 | JOHANSSON E, KOLMODIN-HEDMAN B, KÄLLSTRÖM E, et al.. IgE-mediated sensitization to predatory mites in Swedish greenhouse workers[J]. Allergy, 2003, 58(4): 337-341. |
| 22 | WANG S Y, HERRERA-BALANDRANO D D, WANG Y X, et al.. Biocontrol ability of the Bacillus amyloliquefaciens group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the management of fungal postharvest diseases: a review[J]. J. Agric. Food Chem., 2022, 70(22): 6591-6616. |
| 23 | GLARE T, CARADUS J, GELERNTER W, et al.. Have biopesticides come of age?[J]. Trends Biotechnol., 2012, 30(5): 250-258. |
| 24 | Intelligence Mordor. Microbial pesticides market size & share analysis-growth trends & forecasts (2024-2029)[DB/OL]. Mordor Intelligence, 2024[2024-07-28]. . |
| 25 | 李友顺,白小宁,李富根,等.2023年及近年我国农药登记情况和特点分析[J].农药科学与管理,2024,45(2):10-19+28. |
| LI Y S, BAI X N, LI F G, et al.. Analysis on the situation and characteristics of pesticide registration in China in 2023 and recent years[J]. Pestic. Sci. Adm., 2024, 45(2): 10-19, 28. | |
| 26 | VAN DEN B R. Biological control of insects [J]. Annu. Rev. Ecol. Syst., 1971: 45-66. |
| 27 | USMAN M, WAKIL W, SHAPIRO-ILAN D I. Entomopathogenic nematodes as biological control agent against Bactrocera zonata and Bactrocera dorsalis (Diptera: Tephritidae)[J/OL]. Biol. Contr., 2021, 163: 104706[2024-07-28]. . |
| 28 | CULSHAW-MAURER M, SIH A, ROSENHEIM J A. Bugs scaring bugs: enemy-risk effects in biological control systems[J]. Ecol. Lett., 2020, 23(11): 1693-1714. |
| 29 | VENKANNA Y, SUROSHE S S, DAHUJA A. Non-consumptive effects of the zigzag ladybird beetle, Cheilomenes sexmaculata (Fab.) on its prey, the cotton aphid, Aphis gossypii Glover[J]. Biocontrol Sci. Technol., 2021, 31(11): 1204-1219. |
| 30 | GRASS I, LEHMANN K, THIES C, et al.. Insectivorous birds disrupt biological control of cereal aphids[J]. Ecology, 2017, 98(6): 1583-1590. |
| 31 | JI R, SIMPSON S J, YU F, et al.. Diets of migratory rosy starlings (Passeriformes: Sturnidae) and their effects on grasshoppers: Implications for a biological agent for insect pests[J]. Biol. Contr., 2008, 46(3): 547-551. |
| 32 | LITWIN A, NOWAK M, RÓŻALSKA S. Entomopathogenic fungi: unconventional applications[J]. Rev. Environ. Sci. Bio/Technol., 2020, 19(1): 23-42. |
| 33 | SHAH P A, PELL J K. Entomopathogenic fungi as biological control agents[J]. Appl. Microbiol. Biotechnol., 2003, 61(5-6): 413-423. |
| 34 | GIBSON D M, DONZELLI B G, KRASNOFF S B, et al.. Discovering the secondary metabolite potential encoded within entomopathogenic fungi[J]. Nat. Prod. Rep., 2014, 31(10): 1287-1305. |
| 35 | AZIZOGLU U, JOUZANI G S, YILMAZ N, et al.. Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: a review[J/OL]. Sci. Total Environ., 2020, 734: 139169[2024-07-28]. . |
| 36 | BRAVO A, LIKITVIVATANAVONG S, GILL S S, et al.. Bacillus thuringiensis: a story of a successful bioinsecticide[J]. Insect Biochem. Mol. Biol., 2011, 41(7): 423-431. |
| 37 | RUIU L. Insect pathogenic bacteria in integrated pest management[J]. Insects, 2015, 6(2): 352-367. |
| 38 | LI S, ZHANG N, ZHANG Z, et al.. Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation[J]. Biol. Fertil. Soils, 2013, 49(3): 295-303. |
| 39 | SEGARRA G, CASANOVA E, AVILÉS M, et al.. Trichoderma asperellum strain T34 controls Fusarium wilt disease in tomato plants in soilless culture through competition for iron[J]. Microb. Ecol., 2010, 59(1): 141-149. |
| 40 | DING Y, LIU F, YANG J, et al.. Isolation and identification of Bacillus mojavensis YL-RY0310 and its biocontrol potential against Penicillium expansum and patulin in apples[J/OL]. Biol. Contr., 2023, 182: 105239[2024-07-28]. . |
| 41 | RABARI A, RUPARELIA J, JHA C K, et al.. Articulating beneficial rhizobacteria-mediated plant defenses through induced systemic resistance: a review[J]. Pedosphere, 2023, 33(4): 556-566. |
| 42 | SRINIVASAN K, MATHIVANAN N. Biological control of sunflower necrosis virus disease with powder and liquid formulations of plant growth promoting microbial consortia under field conditions[J]. Biol. Contr., 2009, 51(3): 395-402. |
| 43 | SILVA H A ODA, TEIXEIRA W D, BORGES Á V, et al.. Biocontrol of potato early blight and suppression of Alternaria grandis sporulation by Clonostachys spp.[J]. Plant Pathol., 2021, 70(7): 1677-1685. |
| 44 | WANG R, AN X, LV Y, et al.. Trichoderma asperellum GD040 upregulates defense-related genes and reduces lesion size in Coffea canephora leaves inoculated with Colletotrichum cairnsense [J/OL]. Biol. Contr., 2023, 181: 105213[2024-07-28]. . |
| 45 | GONTHIER J, ARNÓ J, ROMEIS J, et al.. Few indirect effects of baculovirus on parasitoids demonstrate high compatibility of biocontrol methods against Tuta absoluta [J]. Pest Manag. Sci., 2023, 79(4): 1431-1441. |
| 46 | BAI J, LIU Y, LIU M, et al.. Application of phage therapy against red-fleshed kiwifruit canker[J/OL]. Biol. Contr., 2022, 169: 104893[2024-07-28]. . |
| 47 | LIU L, GALILEYA MEDISON R, ZHENG T W, et al.. Biocontrol potential of Bacillus amyloliquefaciens YZU-SG146 from Fraxinus hupehensis against Verticillium wilt of cotton[J/OL]. Biol. Contr., 2023, 183: 105246[2024-07-28]. . |
| 48 | MSIMBIRA L A, JAISWAL S K, DAKORA F D. Identification and characterization of phages parasitic on bradyrhizobia nodulating groundnut (Arachis hypogaea L.) in South Africa[J]. Appl. Soil Ecol., 2016, 108: 334-340. |
| 49 | SITU J, ZHENG L, XU D, et al.. Screening of effective biocontrol agents against postharvest Litchi downy blight caused by Peronophythora litchii [J/OL]. Postharvest Biol. Technol., 2023, 198: 112249[2024-07-28]. . |
| 50 | OH H S, LEE Y H. A target-site-specific screening system for antifungal compounds on appressorium formation in Magnaporthe grisea [J]. Phytopathology, 2000, 90(10): 1162-1168. |
| 51 | KJELDGAARD B, NEVES A R, FONSECA C, et al.. Quantitative high-throughput screening methods designed for identification of bacterial biocontrol strains with antifungal properties[J/OL]. Microbiol. Spectr., 2022, 10(2): e0143321[2024-07-28]. . |
| 52 | HAN S H, KANG B R, LEE J H, et al.. Isolation and characterization of oligotrophic bacteria possessing induced systemic disease resistance against plant pathogens[J]. Plant Pathol. J., 2012, 28(1): 68-74. |
| 53 | SURACHAT K, KANTACHOTE D, DEACHAMAG P, et al.. In silico genomic analysis of Rhodopseudomonas palustris strains revealed potential biocontrol agents and crop yield enhancers[J/OL]. Biol. Contr., 2022, 176: 105085[2024-07-28]. . |
| 54 | MEDINI D, DONATI C, TETTELIN H, et al.. The microbial pan-genome[J]. Curr. Opin. Genet. Dev., 2005, 15(6): 589-594. |
| 55 | HANDELSMAN J. Metagenomics: application of genomics to uncultured microorganisms[J]. Microbiol. Mol. Biol. Rev., 2004, 68(4): 669-685. |
| 56 | POMPANON F, DEAGLE B E, SYMONDSON W O, et al.. Who is eating what: diet assessment using next generation sequencing[J]. Mol. Ecol., 2012, 21(8): 1931-1950. |
| 57 | MATA V A, SILVA L P D A, VERÍSSIMO J, et al.. Combining DNA metabarcoding and ecological networks to inform conservation biocontrol by small vertebrate predators[J/OL]. Ecol. Appl., 2021, 31(8): e02457[2024-07-28]. . |
| 58 | YANG Q, ZHANG X, SOLAIRA J D, et al.. Transcriptomic analyses reveal robust changes in the defense response of apples induced by Hannaella sinensis [J/OL]. Biol. Contr., 2023, 182: 105237[2024-07-28]. . |
| 59 | POVEDA J, EUGUI D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture[J/OL]. Biol. Contr., 2022, 176: 105100[2024-07-28]. . |
| 60 | BAYSAL O, LAI D, XU H H, et al.. A proteomic approach provides new insights into the control of soil-borne plant pathogens by Bacillus species[J/OL]. PLoS One, 2013, 8(1): e53182[2024-07-28]. . |
| 61 | GU N, ZHANG X, GU X, et al.. Proteomic analysis reveals the mechanisms involved in the enhanced biocontrol efficacy of Rhodotorula mucilaginosa induced by chitosan[J/OL]. Biol. Contr., 2020, 149: 104325[2024-07-28]. . |
| 62 | CHEN J, WANG X, TANG D, et al.. Oxidative stress adaptation improves the heat tolerance of Pseudomonas fluorescens SN15-2[J/OL]. Biol. Contr., 2019, 138: 104070[2024-07-28]. . |
| 63 | LI K, CHENG K, WANG H, et al.. Disentangling leaf-microbiome interactions in Arabidopsis thaliana by network mapping[J/OL]. Front. Plant Sci., 2022, 13: 996121[2024-07-28]. . |
| 64 | SARAVANAN A, KUMAR P S, KARISHMA S, et al.. A review on biosynthesis of metal nanoparticles and its environmental applications[J/OL]. Chemosphere, 2021, 264(Pt 2): 128580[2024-07-28]. . |
| 65 | SWAT I, VERMA R, CHAUHAN A, et al.. Antimicrobial potential of ag-doped ZnO nanostructure synthesized by the green method using Moringa oleifera extract[J/OL]. J. Environ. Chem. Eng., 2020, 8(3): 103730[2024-07-28]. . |
| 66 | MOUSTAFA S M N, TAHA R H. Mycogenic nano-complex for plant growth promotion and bio-control of Pythium aphanidermatum [J/OL]. Plants (Basel), 2021, 10(9): 1858[2024-07-28]. . |
| 67 | DJAYA L, HERSANT I, ISTIFADAH N, et al.. In vitro study of plant growth promoting rhizobacteria (PGPR) and endophytic bacteria antagonistic to Ralstonia solanacearum formulated with graphite and silica nano particles as a biocontrol delivery system (BDS)[J/OL]. Biocatal. Agric. Biotechnol., 2019, 19: 101153[2024-07-28]. . |
| 68 | JOGAIAH S, SATAPUTE P, DE BRITTO S, et al.. Exogenous priming of chitosan induces upregulation of phytohormones and resistance against cucumber powdery mildew disease is correlated with localized biosynthesis of defense enzymes[J]. Int. J. Biol. Macromol., 2020, 162: 1825-1838. |
| 69 | FALSINI S, CLEMENTE I, PAPINI A, et al.. When sustainable nanochemistry meets agriculture: lignin nanocapsules for bioactive compound delivery to plantlets[J]. ACS Sustainable Chem. Eng., 2019, 7(24): 19935-19942. |
| 70 | COLLIER T, VAN STEENWYK R. A critical evaluation of augmentative biological control[J]. Biol. Contr., 2004, 31(2): 245-256. |
| 71 | WAJNBERG E, ROITBERG B D, BOIVIN G. Using optimality models to improve the efficacy of parasitoids in biological control programmes[J]. Entomol. Exp. Appl., 2016, 158(1): 2-16. |
| 72 | PLOUVIER W N, WAJNBERG E. Improving the efficiency of augmentative biological control with arthropod natural enemies: a modeling approach[J]. Biol. Contr., 2018, 125: 121-130. |
| 73 | BALANZA V, MENDOZA J E, BIELZA P. Variation in susceptibility and selection for resistance to imidacloprid and thiamethoxam in Mediterranean populations of Orius laevigatus [J]. Entomol. Exp. Appl., 2019, 167(7): 626-635. |
| 74 | MENDOZA J E, BALANZA V, CIFUENTES D, et al.. Genetic improvement of Orius laevigatus for better fitness feeding on pollen[J]. J. Pest Sci., 2021, 94(3): 729-742. |
| 75 | BIELZA P, BALANZA V, CIFUENTES D, et al.. Challenges facing arthropod biological control: identifying traits for genetic improvement of predators in protected crops[J]. Pest Manag. Sci., 2020, 76(11): 3517-3526. |
| 76 | WANG Y, LUO Y, SUI Y, et al.. Exposure of Candida oleophila to sublethal salt stress induces an antioxidant response and improves biocontrol efficacy[J]. Biol. Contr., 2018, 127: 109-115. |
| 77 | ZHU X, WANG Y, WANG X, et al.. Exogenous regulators enhance the yield and stress resistance of chlamydospores of the biocontrol agent Trichoderma harzianum T4[J/OL]. J. Fungi (Basel), 2022, 8(10): 1017[2024-07-28]. . |
| 78 | CABREFIGA J, FRANCÉS J, MONTESINOS E, et al.. Improvement of fitness and efficacy of a fire blight biocontrol agent via nutritional enhancement combined with osmoadaptation[J]. Appl. Environ. Microbiol., 2011, 77(10): 3174-3181. |
| 79 | EL-SHARKAWY H H A, RASHAD Y M, ELAZAB N T. Synergism between Streptomyces viridosporus HH1 and Rhizophagus irregularis effectively induces defense responses to Fusarium wilt of pea and improves plant growth and yield[J/OL]. J. Fungi (Basel), 2022, 8(7): 683[2024-07-28]. . |
| 80 | DU Y, XIA Y, JIN K. Enhancing the biocontrol potential of the entomopathogenic fungus in multiple respects via the overexpression of a transcription factor gene MaSom1 [J/OL]. J. Fungi (Basel), 2022, 8(2): 105[2024-07-28]. . |
| 81 | LIU D, SUN H, MA H. Deciphering microbiome related to rusty roots of Panax ginseng and evaluation of antagonists against pathogenic Ilyonectria [J/OL]. Front. Microbiol., 2019, 10: 1350[2024-07-28]. . |
| 82 | LIU S, MOON C D, ZHENG N, et al.. Opportunities and challenges of using metagenomic data to bring uncultured microbes into cultivation[J/OL]. Microbiome, 2022, 10(1): 76[2024-07-28]. . |
| 83 | BIGGS M B, CRAIG K, GACHANGO E, et al.. Genomics- and machine learning-accelerated discovery of biocontrol bacteria[J]. Phytobiomes J., 2021, 5(4): 452-463. |
| 84 | DE CARVALHO B C M V, BARBOSA E T, DE SOUSA O M I, et al.. Trichoderma harzianum marker-free strain construction based on efficient CRISPR/Cas9 recyclable system: a helpful tool for the study of biological control agents[J/OL]. Biol. Contr., 2023, 184: 105281[2024-07-28]. . |
| [1] | 吴必聪, 焦博, 张雨, 郭鑫, 张誉, 罗晓红, 代蕾, 王强. 料液比对搅拌型UHT核桃酸奶品质特性的影响[J]. 生物技术进展, 2024, 14(4): 640-648. |
| [2] | 徐畅, 刘天一, 刘文佳, 张俐敏, 莫继先. 微生物胞外多糖的来源、生物合成及功能研究进展[J]. 生物技术进展, 2024, 14(3): 368-376. |
| [3] | 孙佳琪, 郭嘉, 张闯, 柳青, 王梓钰, 夏涵超, 钱步轩, 赵方方, 王棋, 刘剑锋, 刘相国. 亚磷酸脱氢酶在基因工程改造微生物和植物中的研究进展[J]. 生物技术进展, 2024, 14(2): 173-181. |
| [4] | 刘鑫, 李华, 齐磊, 杨利霞, 于月欣, 徐兰举. 重组胶原蛋白妇科凝胶对大鼠宫颈炎的治疗作用[J]. 生物技术进展, 2024, 14(1): 42-47. |
| [5] | 齐航宇, 都婷婷, 高权新, 唐琼英, 杨国梁, 易少奎. 虾蟹类动物社会等级研究进展[J]. 生物技术进展, 2023, 13(6): 827-836. |
| [6] | 方靖靖, 黄昆仑, 仝涛. 孤独症谱系障碍实验模型研究进展[J]. 生物技术进展, 2023, 13(4): 509-523. |
| [7] | 马云鹏, 朱静, 崔兴华. 基于机器学习的微生物溶解有机碳含量估测[J]. 生物技术进展, 2023, 13(4): 645-653. |
| [8] | 邱思元, 徐晶雪, 段育阳, 赵金玉, 赵文婧, 张莉欣, 任国领. 甘露糖赤藓糖醇脂生产及应用研究进展[J]. 生物技术进展, 2023, 13(2): 210-219. |
| [9] | 郝捷, 季嫱, 李力群, 郑超, 吴娜, 吴晗, 李选文, 孙志康. 生物酶和微生物技术改善烟叶香气的研究进展[J]. 生物技术进展, 2022, 12(6): 817-824. |
| [10] | 周水娟, 郭忠建. 双分子荧光互补技术在动物病毒中的研究进展[J]. 生物技术进展, 2022, 12(6): 825-836. |
| [11] | 范蓓, 王园园, 赵云霞, 江魁, 王斌. 免疫系统人源化小鼠模型构建技术探讨[J]. 生物技术进展, 2022, 12(6): 922-928. |
| [12] | 刘培敏, 罗金萍, 高权新. 水产养殖环境微生物研究进展[J]. 生物技术进展, 2022, 12(5): 690-695. |
| [13] | 李力群, 孙志康, 郝捷, 季嫱, 李选文, 吴晗, 吴娜, 郑超, 杨婧. 果胶酶生产及工业应用进展[J]. 生物技术进展, 2022, 12(4): 549-558. |
| [14] | 王濛, 仪杨, 孙梦婷, 刘梓嘉, 姜雪, 马晨, 宋怡菲, 谢飞. 富氢水和富氢生理盐水生物医学研究进展——动物实验[J]. 生物技术进展, 2022, 12(3): 332-343. |
| [15] | 余卉茹, 纪艺, 彭城, 徐晓丽, 汪小福, 孙梅好, 陈笑芸. 肉类掺假检测技术研究进展[J]. 生物技术进展, 2022, 12(2): 213-221. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部