


生物技术进展 ›› 2024, Vol. 14 ›› Issue (3): 388-398.DOI: 10.19586/j.2095-2341.2024.0034
• 进展评述 • 上一篇
收稿日期:2024-02-29
接受日期:2024-03-25
出版日期:2024-05-25
发布日期:2024-06-18
通讯作者:
吕新星
作者简介:李鸿博 E-mail: li463195983@qq.com;
基金资助:
Hongbo LI( ), Zhuyue CHEN, Xinxing LYU(
), Zhuyue CHEN, Xinxing LYU( )
)
Received:2024-02-29
Accepted:2024-03-25
Online:2024-05-25
Published:2024-06-18
Contact:
Xinxing LYU
摘要:
衰老是机体随着时间推移而发生的不可抗拒的自然变化,表现为生物体形态结构的改变和生理功能的衰退,同时伴随着多种老年性疾病的发生。亚精胺作为天然的多胺类物质,在抑制机体衰老进程中发挥着重要作用。最近的研究表明,亚精胺通过激活细胞自噬,清除受损的线粒体,干预脂肪代谢和调节细胞周期等方式,清除衰老细胞,维持组织微环境稳定,抑制衰老相关疾病的发生和进展。系统地阐述了亚精胺的体内和体外的合成过程,缓解细胞衰老的分子机制,以及在减缓机体衰老生理过程和多种衰老相关疾病中的治疗作用,以期为衰老相关疾病的转归与临床治疗提供参考。
中图分类号:
李鸿博, 陈朱玥, 吕新星. 亚精胺缓解细胞衰老及衰老相关疾病的研究进展[J]. 生物技术进展, 2024, 14(3): 388-398.
Hongbo LI, Zhuyue CHEN, Xinxing LYU. Recent Progress on Spermidine Alleviating Cell Senescence and Aging-related Diseases[J]. Current Biotechnology, 2024, 14(3): 388-398.
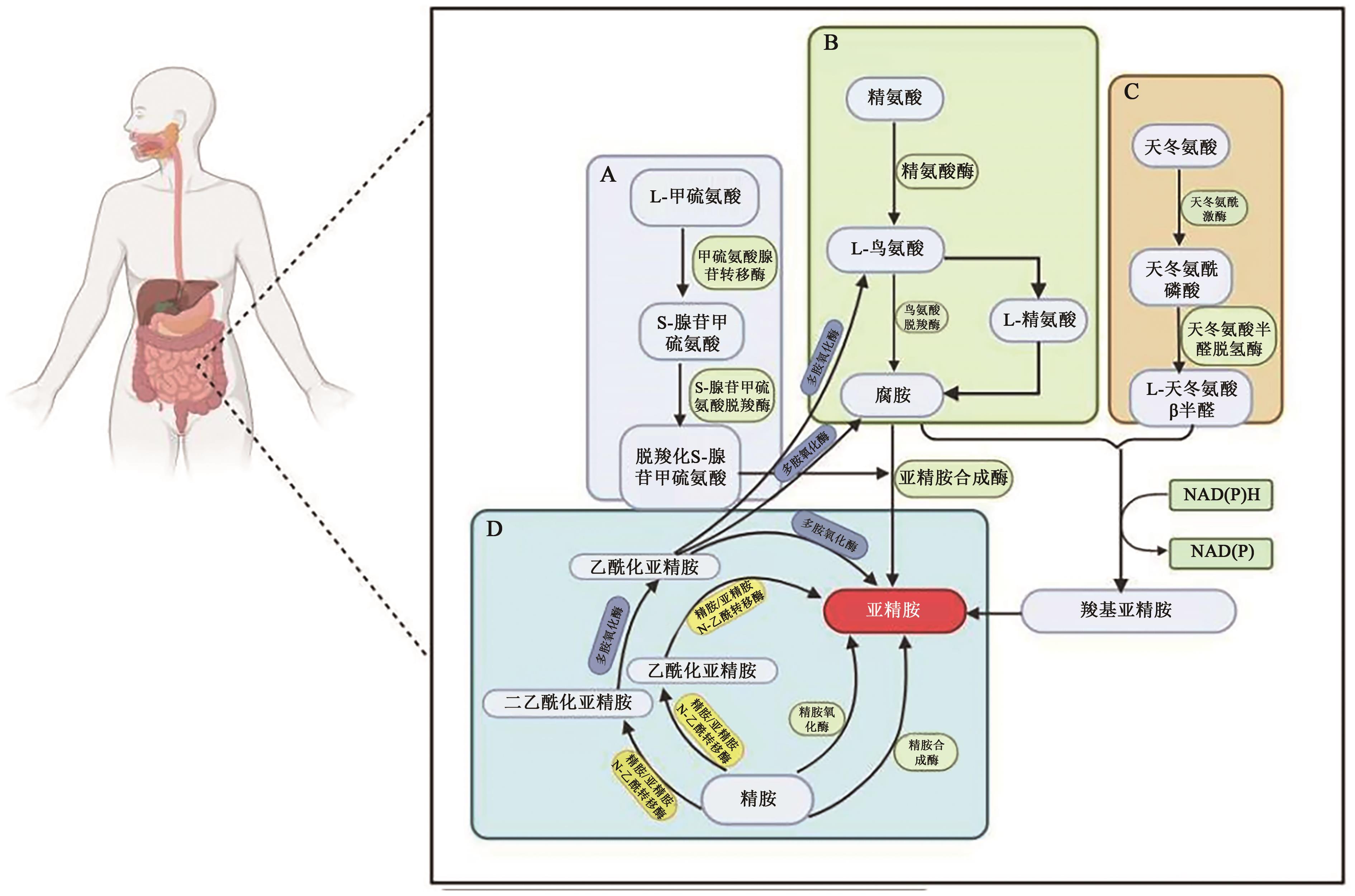
图2 哺乳动物体内的亚精胺合成图A:S-腺苷甲硫氨酸合成途径(Sam合成途径);B:以鸟氨酸为原料生成腐胺的过程;C:天冬氨酸半醛合成途径(Asa合成途径);D:亚精胺与精胺的转化关系
Fig. 2 The process of spermidine synthesis in mammalian cells
| 时间 | 菌株/平台 | 方法 | 底物 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 2017年 | Saccharomyces cerevisiae | 代谢工程 | 葡萄糖/木糖 | 224.0 mg·L-1 | [ |
| 2020年 | Bacillus amyloliquefaciens | 代谢工程 | 果糖/木糖 | 227.4 mg·L-1 | [ |
| 2021年 | Saccharomyces cerevisiae | 代谢工程 | 葡萄糖 | 2.3 g·L-1 | [ |
| 2021年 | 酶(MAT+SAMDC) | 酶催化 | Sam和腐胺 | 3.7 g·L-1 | [ |
表1 不同种类的亚精胺微生物发酵法
Table 1 List of different types of microbial fermentation methods for spermidine
| 时间 | 菌株/平台 | 方法 | 底物 | 产量 | 参考文献 |
|---|---|---|---|---|---|
| 2017年 | Saccharomyces cerevisiae | 代谢工程 | 葡萄糖/木糖 | 224.0 mg·L-1 | [ |
| 2020年 | Bacillus amyloliquefaciens | 代谢工程 | 果糖/木糖 | 227.4 mg·L-1 | [ |
| 2021年 | Saccharomyces cerevisiae | 代谢工程 | 葡萄糖 | 2.3 g·L-1 | [ |
| 2021年 | 酶(MAT+SAMDC) | 酶催化 | Sam和腐胺 | 3.7 g·L-1 | [ |
| 1 | LEI J, JIANG X, LI W, et al.. Exosomes from antler stem cells alleviate mesenchymal stem cell senescence and osteoarthritis[J]. Protein Cell, 2022, 13(3): 220-226. |
| 2 | LI Z, ZHOU D, ZHANG D, et al.. Folic acid inhibits aging-induced telomere attrition and apoptosis in astrocytes in vivo and in vitro [J]. Cereb. Cortex, 2022, 32(2): 286-297. |
| 3 | BHARATH L P, AGRAWAL M, MCCAMBRIDGE G, et al.. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation[J]. Cell Metab., 2020, 32(1): 44-55. |
| 4 | MOSSAD O, BATUT B, YILMAZ B, et al.. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N(6)-carboxymethyllysine[J]. Nat. Neurosci., 2022, 25(3): 295-305. |
| 5 | TRACY T E, MADERO-PÉREZ J, SWANEY D L, et al.. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration[J]. Cell, 2022, 185(4): 712-728. |
| 6 | SAIKI S, SASAZAWA Y, FUJIMAKI M, et al.. A metabolic profile of polyamines in parkinson disease: a promising biomarker[J]. Ann. Neurol., 2019, 86(2): 251-263. |
| 7 | LI J, ZHANG L, XIONG J, et al.. Polyamines disrupt the KaiABC oscillator by inducing protein denaturation[J/OL]. Molecules, 2019, 24(18): 3351[2024-04-12]. . |
| 8 | MUÑOZ-ESPARZA N C, COSTA-CATALA J, COMAS-BASTÉ O, et al.. Occurrence of polyamines in foods and the influence of cooking processes[J/OL]. Foods, 2021, 10(8): 1752[2024-04-12]. . |
| 9 | OSBORNE D L, SEIDEL E R. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation[J]. Am. J. Physiol., 1990, 258(4 Pt 1): 576-584. |
| 10 | MILLER-FLEMING L, OLIN-SANDOVAL V, CAMPBELL K, et al.. Remaining mysteries of molecular biology: the role of polyamines in the cell[J]. J. Mol. Biol., 2015, 427(21): 3389-3406. |
| 11 | SCALABRINO G, FERIOLI M E. Polyamines in mammalian ageing: an oncological problem, too? A review[J]. Mech. Ageing Dev., 1984, 26(2-3): 149-164. |
| 12 | IGARASHI K, KASHIWAGI K. Modulation of cellular function by polyamines[J]. Int. J. Biochem. Cell Biol., 2010, 42(1): 39-51. |
| 13 | TAIT G H. A new pathway for the biosynthesis of spermidine[J]. Biochem. Soc. Trans., 1976, 4(4): 610-612. |
| 14 | QIAN Z G, XIA X X, LEE S Y. Metabolic engineering of Escherichia coli for the production of putrescine: a four carbon diamine[J]. Biotechnol. Bioeng., 2009, 104(4): 651-662. |
| 15 | DAVIS R H, MORRIS D R, COFFINO P. Sequestered end products and enzyme regulation: the case of ornithine decarboxylase[J]. Microbiol. Rev., 1992, 56(2): 280-290. |
| 16 | PEGG A E. Mammalian polyamine metabolism and function[J]. IUBMB Life, 2009, 61(9): 880-894. |
| 17 | KIM S K, JIN Y S, CHOI I G, et al.. Enhanced tolerance of Saccharomyces cerevisiae to multiple lignocellulose-derived inhibitors through modulation of spermidine contents[J]. Metab. Eng., 2015, 29: 46-55. |
| 18 | LIU Y, GUO X, WANG X, et al.. A two-enzyme cascade system for the bio-production of spermidine from putrescine[J/OL]. Mol. Catal., 2021, 504: 111439[2024-04-12]. . |
| 19 | LI L, ZOU D, JI A, et al.. Multilevel metabolic engineering of Bacillus amyloliquefaciens for production of the platform chemical putrescine from sustainable biomass hydrolysates[J]. ACS Sustain. Chem. Eng., 2020, 8(5): 2147-2157. |
| 20 | HANFREY C C, PEARSON B M, HAZELDINE S, et al.. Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota[J]. J. Biol. Chem., 2011, 286(50): 43301-43312. |
| 21 | QIN J, KRIVORUCHKO A, JI B, et al.. Engineering yeast metabolism for the discovery and production of polyamines and polyamine analogues[J]. Nat. Catal., 2021, 4: 498-509. |
| 22 | DUDLEY H W, ROSENHEIM M C, ROSENHEIM O. The chemical constitution of spermine. I. the isolation of spermine from animal tissues, and the preparation of its salts[J]. Biochem. J., 1924, 18(6): 1263-1272. |
| 23 | EISENBERG T, KNAUER H, SCHAUER A, et al.. Induction of autophagy by spermidine promotes longevity[J]. Nat. Cell Biol., 2009, 11(11): 1305-1314. |
| 24 | MADEO F, EISENBERG T, BÜTTNER S, et al.. Spermidine: a novel autophagy inducer and longevity elixir[J]. Autophagy, 2010, 6(1): 160-162. |
| 25 | PIETROCOLA F, LACHKAR S, ENOT D P, et al.. Spermidine induces autophagy by inhibiting the acetyltransferase EP300[J]. Cell Death Differ., 2015, 22(3): 509-516. |
| 26 | PULESTON D J, BUCK M D, KLEIN GELTINK R I, et al.. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation[J]. Cell Metab., 2019, 30(2): 352-363. |
| 27 | KEE K, FOSTER B A, MERALI S, et al.. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice[J]. J. Biol. Chem., 2004, 279(38): 40076-40083. |
| 28 | JELL J, MERALI S, HENSEN M L, et al.. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA[J]. J. Biol. Chem., 2007, 282(11): 8404-8413. |
| 29 | PEGG A E. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator[J]. Am. J. Physiol. Endocrinol. Metab., 2008, 294(6): 995-1010. |
| 30 | LIANG Y, PIAO C, BEUSCHEL C B, et al.. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction[J/OL]. Cell Rep., 2021, 35(2): 108941[2024-04-12]. . |
| 31 | ZHANG H, ALSALEH G, FELTHAM J, et al.. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence[J]. Mol. Cell, 2019, 76(1): 110-125. |
| 32 | SCHROEDER S, HOFER S J, ZIMMERMANN A, et al.. Dietary spermidine improves cognitive function[J/OL]. Cell Rep., 2021, 35(2): 108985[2024-04-12]. . |
| 33 | ALSALEH G, PANSE I, SWADLING L, et al.. Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses[J/OL]. eLife, 2020, 9: e57950[2024-04-12]. . |
| 34 | 王珍,杨洛,廖敏,等.mTOR信号通路在糖尿病肾病发病机制中的研究进展[J].生物技术进展,2021,11(3):316-321. |
| WANG Z, YANG L, LIAO M, et al.. Research progress of mTOR pathway in pathogenesis of diabetic nephropathy[J]. Curr. Biotechnol., 2021, 11(3): 316-321. | |
| 35 | 郭婧雅,张萍,赵雨菡,等.肥胖诱导的骨骼肌萎缩机制研究进展[J].生物技术进展,2022,12(6):861-868. |
| GUO J Y, ZHANG P, ZHAO Y H, et al.. Advances on the mechanism of obesity-induced skeletal muscle atrophy[J]. Curr. Biotechnol., 2022, 12(6): 861-868. | |
| 36 | YAN J, YAN J Y, WANG Y X, et al.. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway[J]. Br. J. Pharmacol., 2019, 176(17): 3126-3142. |
| 37 | PARTRIDGE L, FUENTEALBA M, KENNEDY B K. The quest to slow ageing through drug discovery[J]. Nat. Rev. Drug Discov., 2020, 19(8): 513-532. |
| 38 | QI Y, QIU Q, GU X, et al.. ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts[J/OL]. Sci. Rep., 2016, 6: 24700[2024-04-12. . |
| 39 | 张维,王红芳,胥保华.生物衰老的主要分子机制概述[J].生物技术进展,2023,13(2):228-233. |
| ZHANG W, WANG H F, XU B H. Overview of the main molecular mechanisms of biological aging[J]. Curr. Biotechnol., 2023, 13(2): 228-233. | |
| 40 | SINGH S, KUMAR R, GARG G, et al.. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging[J]. Biogerontology, 2021, 22(1): 35-47. |
| 41 | D'ADAMO S, CETRULLO S, GUIDOTTI S, et al.. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes[J]. Free. Radic. Biol. Med., 2020, 153: 159-172. |
| 42 | JIANG D, WANG X, ZHOU X, et al.. Spermidine alleviating oxidative stress and apoptosis by inducing autophagy of granulosa cells in Sichuan white geese[J/OL]. Poult. Sci., 2023, 102(9): 102879[2024-04-12]. . |
| 43 | ZHENG Z, WANG Z G, CHEN Y, et al.. Spermidine promotes nucleus pulposus autophagy as a protective mechanism against apoptosis and ameliorates disc degeneration[J]. J. Cell. Mol. Med., 2018, 22(6): 3086-3096. |
| 44 | AL-HABSI M, CHAMOTO K, MATSUMOTO K, et al.. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice[J/OL]. Science, 2022, 378(6618): eabj3510[2024-04-12]. . |
| 45 | CARRICHE G M, ALMEIDA L, STÜVE P, et al.. Regulating T-cell differentiation through the polyamine spermidine[J]. J. Allergy Clin. Immunol., 2021, 147(1): 335-348. |
| 46 | LI X, ZHOU X, LIU X, et al.. Spermidine protects against acute kidney injury by modulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway[J/OL]. Mol. Med., 2022, 28(1): 103[2024-04-12]. . |
| 47 | DU FOSSÉ N A, VAN DER HOORN M P, VAN LITH J M M, et al.. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: a systematic review and meta-analysis[J]. Hum. Reprod. Update, 2020, 26(5): 650-669. |
| 48 | ZHANG Y, BAI J, CUI Z, et al.. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging[J]. Nat. Aging, 2023, 3(11): 1372-1386. |
| 49 | 徐磊. 2018年中国生理学会运动生理学专业委员会会议暨"科技创新与运动生理学"学术研讨会论文集,北京体育大学, 2018 [C]//武汉:武汉体育学院研究生院,2018. |
| 50 | 范晶晶.第四届全国运动生理与生物化学学术会议—运动·体质·健康论文摘要汇编[C]//中国体育科学学会运动生理与生物化学分会,2016. |
| 51 | 王誉西.亚精胺通过促进细胞自噬流抑制人脐静脉内皮细胞衰老[D].广州:南方医科大学,2019. |
| 52 | FU J, PENG L, TAO T, et al.. Regulatory roles of the miR-200 family in neurodegenerative diseases[J/OL]. Biomed. Pharmacother., 2019, 119: 109409[2024-04-12]. . |
| 53 | GUO F, LIU X, CAI H, et al.. Autophagy in neurodegenerative diseases: pathogenesis and therapy[J]. Brain Pathol., 2018, 28(1): 3-13. |
| 54 | METAXAKIS A, PLOUMI C, TAVERNARAKIS N. Autophagy in age-associated neurodegeneration[J/OL]. Cells, 2018, 7(5): 37[2024-04-12]. . |
| 55 | BÜTTNER S, BROESKAMP F, SOMMER C, et al.. Spermidine protects against α-synuclein neurotoxicity[J]. Cell Cycle Georget. Tex, 2014, 13(24): 3903-3908. |
| 56 | FAN J, YANG X, LI J, et al.. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway[J]. Oncotarget, 2017, 8(11): 17475-17490. |
| 57 | CUANALO-CONTRERAS K, MORENO-GONZALEZ I. Natural products as modulators of the proteostasis machinery: implications in neurodegenerative diseases[J/OL]. Int. J. Mol. Sci., 2019, 20(19): 4666[2024-04-12]. . |
| 58 | PHADWAL K, KURIAN D, SALAMAT M K F, et al.. Spermine increases acetylation of tubulins and facilitates autophagic degradation of prion aggregates[J/OL]. Sci. Rep., 2018, 8(1): 10004[2024-04-12]. . |
| 59 | VIJAYAN B, RAJ V, NANDAKUMAR S, et al.. Spermine protects alpha-synuclein expressing dopaminergic neurons from manganese-induced degeneration[J]. Cell Biol. Toxicol., 2019, 35(2): 147-159. |
| 60 | GRANCARA S, ZONTA F, OHKUBO S, et al.. Pathophysiological implications of mitochondrial oxidative stress mediated by mitochondriotropic agents and polyamines: the role of tyrosine phosphorylation[J]. Amino Acids, 2015, 47(5): 869-883. |
| 61 | MINOIS N, ROCKENFELLER P, SMITH T K, et al.. Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition[J/OL]. PLoS ONE, 2014, 9(7): e102435[2024-04-12]. . |
| 62 | KISHI A, OHNO M, WATANABE S., polyamine site agonista, attenuates working memory deficits caused by blockade of hippocampal muscarinic receptors and mGluRs in rats[J]. Brain Res., 1998, 793(1-2): 311-314. |
| 63 | VELLOSO N A, DALMOLIN G D, GOMES G M, et al.. Spermine improves recognition memory deficit in a rodent model of Huntington's disease[J]. Neurobiol. Learn. Mem., 2009, 92(4): 574-580. |
| [1] | 黄师, 莫茵茵, 罗绿景, 刘会婷, 陈峥宇, 李根亮. NHP2调控肝癌细胞衰老机制的生物信息学分析[J]. 生物技术进展, 2024, 14(1): 141-148. |
| [2] | 张维, 王红芳, 胥保华. 生物衰老的主要分子机制概述[J]. 生物技术进展, 2023, 13(2): 228-233. |
| [3] | 李玉珍, 朱杰夫, 吴雄飞. PIM1激酶在顺铂诱导的急性肾损伤中的作用[J]. 生物技术进展, 2023, 13(2): 298-304. |
| [4] | 赵云鹏, 张浩林, 熊倩倩, 李雨婷, 王娟. COPII囊泡衣被蛋白SEC24A在巨自噬通路中的功能研究[J]. 生物技术进展, 2022, 12(6): 906-914. |
| [5] | 侯凯耀, 张二飞, 郑李娜, 陈红光, 谢克亮. 富氢液对脓毒症小鼠心肌细胞线粒体自噬的影响[J]. 生物技术进展, 2022, 12(4): 497-502. |
| [6] | 孙卉, 张春义, 姜凌. 辅酶Ⅰ体内代谢调控研究进展[J]. 生物技术进展, 2021, 11(4): 526-534. |
| [7] | 王珍,杨洛,廖敏,郝亚荣. mTOR信号通路在糖尿病肾病发病机制中的研究进展[J]. 生物技术进展, 2021, 11(3): 316-321. |
| [8] | 林晶晶,杨宇丰. 线粒体自噬的调控机制及其在相关疾病中的作用[J]. 生物技术进展, 2019, 9(5): 467-475. |
| [9] | 徐长禄,赵艳红,马艺戈,王冰蕊,王鼎,郭青,佟静媛,高洁,李亚朴,刘金花,石莉红. 利用生物信息学方法解析自噬相关基因在人体红细胞终末分化各阶段的动态表达[J]. 生物技术进展, 2019, 9(3): 271-276. |
| [10] | 白志慧,王彦,张日丽,许维恒,张俊平. 秀丽隐杆线虫免疫衰老指标的建立[J]. 生物技术进展, 2017, 7(3): 230-235. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部