


生物技术进展 ›› 2024, Vol. 14 ›› Issue (4): 566-575.DOI: 10.19586/j.2095-2341.2024.0004
• 进展评述 • 上一篇
收稿日期:2024-01-09
接受日期:2024-05-20
出版日期:2024-07-25
发布日期:2024-08-07
通讯作者:
于正洪
作者简介:胡亚萍 E-mail: 2868240250@qq.com;
基金资助:Received:2024-01-09
Accepted:2024-05-20
Online:2024-07-25
Published:2024-08-07
Contact:
Zhenghong YU
摘要:
mRNA疫苗通过传递编码病原体蛋白的遗传信息信使RNA,激活人体免疫系统产生针对特定病原体的保护性免疫反应。mRNA疫苗的优势在于其快速研发、高度针对性以及可通过结构优化提供长效保护,为未来疫苗研发提供了革命性的新途径。目前,基于mRNA预防病毒传染病的疫苗已经应用于新型冠状病毒感染(corona virus disease 2019,COVID-19)、流感、狂犬病等。总结了mRNA疫苗的研发历程、结构特性以及作用机理,阐述了其在几种特定病毒性疾病中的应用情况、临床效果以及存在的局限性,提出了mRNA疫苗在应对病毒性传染病时所面临的机遇与挑战,并展望了其在未来病毒性传染病防控中的应用前景,以期为mRNA疫苗的研发和应用提供参考。
中图分类号:
胡亚萍, 于正洪. mRNA疫苗预防病毒性传染病的研究现状[J]. 生物技术进展, 2024, 14(4): 566-575.
Yaping HU, Zhenghong YU. Current Status of Research on mRNA Vaccines Against Viral Infectious Diseases[J]. Current Biotechnology, 2024, 14(4): 566-575.
| 疫苗类型 | 设计 | 稳定性 | 安全性 | 免疫原性 | 缺点 |
|---|---|---|---|---|---|
| 不灵活 | 差 | 差 | 较强 | 有毒力恢复的风险 | |
| 不灵活 | 好 | 较好 | 较弱 | 接种剂次多 | |
| 灵活 | 好 | 一般 | 强 | 基因重组风险高 | |
| 灵活 | 好 | 好 | 较弱 | 基因重组风险高 | |
| 灵活 | 差 | 好 | 强 | 长期安全性未知 |
表1 5种疫苗类型的优缺点特征
Table 1 Advantages and disadvantages of five vaccine methods
| 疫苗类型 | 设计 | 稳定性 | 安全性 | 免疫原性 | 缺点 |
|---|---|---|---|---|---|
| 不灵活 | 差 | 差 | 较强 | 有毒力恢复的风险 | |
| 不灵活 | 好 | 较好 | 较弱 | 接种剂次多 | |
| 灵活 | 好 | 一般 | 强 | 基因重组风险高 | |
| 灵活 | 好 | 好 | 较弱 | 基因重组风险高 | |
| 灵活 | 差 | 好 | 强 | 长期安全性未知 |
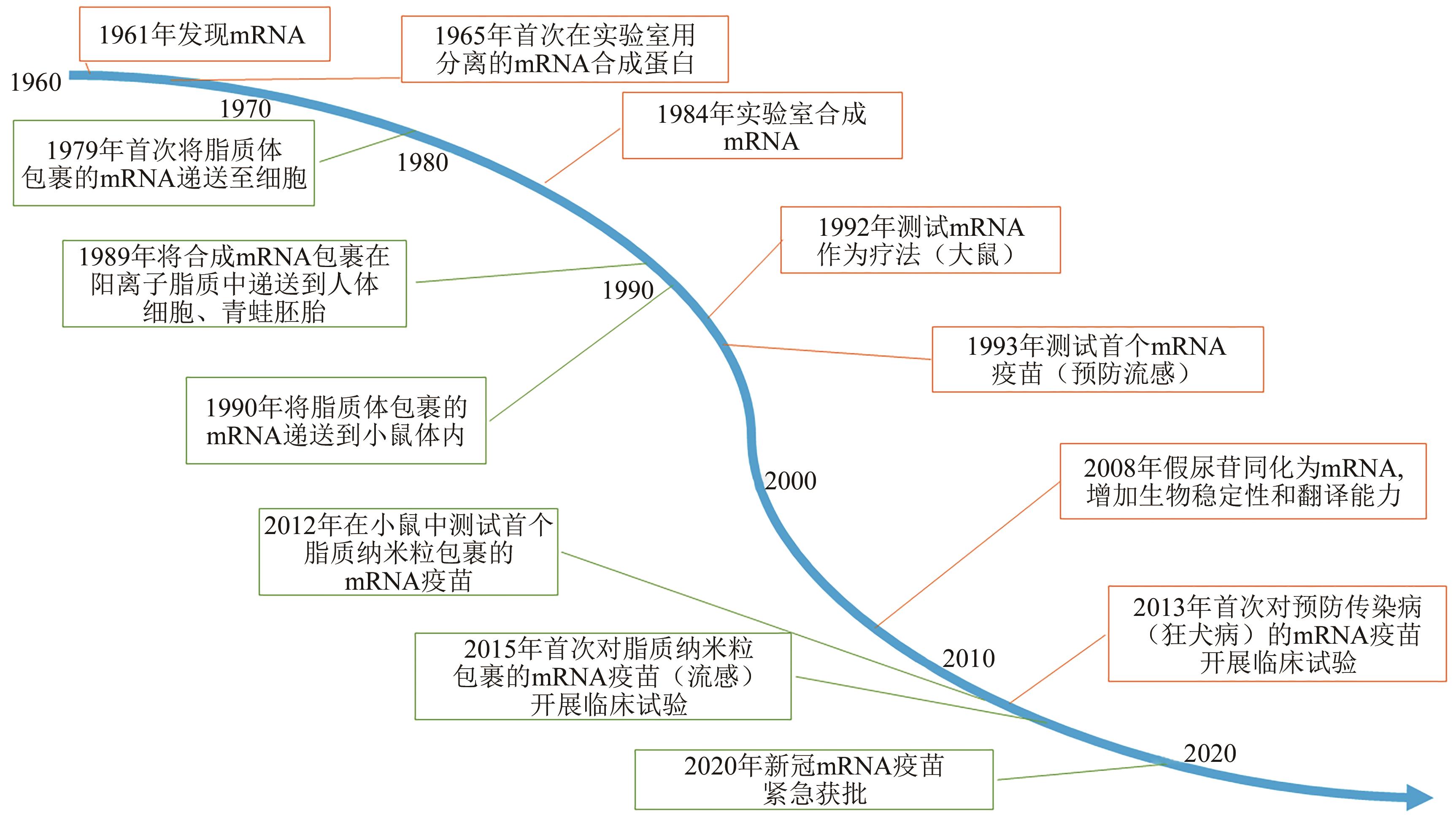
图1 mRNA疫苗取得的科学进展离不开mRNA和递送系统(脂质分子)注:绿色方框代表mRNA的发现和mRNA疫苗研发关键时间点;橙色方框代表加入脂质的mRNA疫苗技术研发关键时间点。
Fig. 1 The scientific progress of mRNA vaccines is inseparable from the mRNA and delivery system (lipid molecules)
| 疫苗名称 | 来源 | 特点 | 效果 | 储存与运输 | 安全性 | 参考文献 |
|---|---|---|---|---|---|---|
| mRNA-1273 | Moderna | 编码封装在新型LNP中的SARS-CoV-2刺突蛋白 | 免疫原性反应在首次疫苗接种后持续至少119 d,且受到给药剂量的极大影响,预防SARS-CoV-5感染的效力达94.5% | -20 ℃ | 无明显安全问题 | [ |
| mRNA BNT162b2 | 辉瑞生物科技(美国纽约) | 采用多功能脂质颗粒系统配制而成,可引发针对SARS-CoV-2刺突蛋白的免疫原性 | 注射第1剂后有52%的效力,第2剂后SARS-CoV-2轻度至重度感染病例的效力为95%,这些疫苗在早期保护方面显示出有希望的结果 | -70 ℃ | [ | |
| CVnCoV | Curevac(德国蒂宾根) | 未经化学修饰,在LNP中配制成mRNA,并编码全长SARS-CoV-2刺突蛋白 | 该疫苗除了诱导特异性 T细胞反应外,还引发与感染者恢复期血清中表现相当的免疫应答 | - | - | [ |
| ARCT-021 | Arcturus Therapeutics(美国加利福尼亚州圣地亚哥) | 利用自转录和复制RNA技术,并在脂质启用和解锁的核酸修饰RNA系统中递送 | 2 μg疫苗在给药60 d后具有增强中和抗体的能力,可诱导强大的CD8 +T细胞和Th1偏向的T辅助 | - | - | [ |
| ARCoV | 军事医学科学院、苏州艾博生物和沃森生物 | 无需冷冻保存,2~8 ℃ |
表3 几种COVID-9 mRNA疫苗比较
Table 3 Comparison of several COVID-9 mRNA vaccines
| 疫苗名称 | 来源 | 特点 | 效果 | 储存与运输 | 安全性 | 参考文献 |
|---|---|---|---|---|---|---|
| mRNA-1273 | Moderna | 编码封装在新型LNP中的SARS-CoV-2刺突蛋白 | 免疫原性反应在首次疫苗接种后持续至少119 d,且受到给药剂量的极大影响,预防SARS-CoV-5感染的效力达94.5% | -20 ℃ | 无明显安全问题 | [ |
| mRNA BNT162b2 | 辉瑞生物科技(美国纽约) | 采用多功能脂质颗粒系统配制而成,可引发针对SARS-CoV-2刺突蛋白的免疫原性 | 注射第1剂后有52%的效力,第2剂后SARS-CoV-2轻度至重度感染病例的效力为95%,这些疫苗在早期保护方面显示出有希望的结果 | -70 ℃ | [ | |
| CVnCoV | Curevac(德国蒂宾根) | 未经化学修饰,在LNP中配制成mRNA,并编码全长SARS-CoV-2刺突蛋白 | 该疫苗除了诱导特异性 T细胞反应外,还引发与感染者恢复期血清中表现相当的免疫应答 | - | - | [ |
| ARCT-021 | Arcturus Therapeutics(美国加利福尼亚州圣地亚哥) | 利用自转录和复制RNA技术,并在脂质启用和解锁的核酸修饰RNA系统中递送 | 2 μg疫苗在给药60 d后具有增强中和抗体的能力,可诱导强大的CD8 +T细胞和Th1偏向的T辅助 | - | - | [ |
| ARCoV | 军事医学科学院、苏州艾博生物和沃森生物 | 无需冷冻保存,2~8 ℃ |
| 1 | MCARTHUR D B. Emerging infectious diseases[J]. Nurs. Clin. N. Am., 2019, 54( 2): 297- 311. |
| 2 | MURPHY F A. Historical perspective: what constitutes discovery (of a new virus)?[J]. Adv. Virol. Res., 2016, 95: 197- 220. |
| 3 | HUI D S C, CHAN P K S. Severe acute respiratory syndrome and coronavirus[J]. Infect. Dis. Clin. N. Am., 2010, 24( 3): 619- 638. |
| 4 | GRAÑA C, GHOSN L, EVRENOGLOU T, et al.. Efficacy and safety of COVID-19 vaccines[J/OL]. Cochrane Db. Syst. Rev., 2022, 12( 12): CD015477[ 2024-06-14]. . |
| 5 | ZHANG C, MARUGGI G, SHAN H, et al.. Advances in mRNA vaccines for infectious diseases[J/OL]. Front. Immunol., 2019, 10: 594[ 2024-06-14]. . |
| 6 | THESS A, GRUND S, MUI B L, et al.. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals[J]. Mol. Ther., 2015, 23( 9): 1456- 1464. |
| 7 | SONG J, WANG Q, WU J, et al.. The influence of fluoride ions on the equilibrium between titanium ions and titanium metal in fused alkali chloride melts[J]. Faraday Discuss., 2016, 190: 421- 432. |
| 8 | BRENNER S, JACOB F, MESELSON M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis[J]. Nature, 1961, 190: 576- 581. |
| 9 | MALONE R W, FELGNER P L, VERMA I M. Cationic liposome-mediated RNA transfection[J]. Proc. Natl. Acad. Sci. USA, 1989, 86( 16): 6077- 6081. |
| 10 | WOLFF J A, MALONE R W, WILLIAMS P . et al .. Direct gene transfer into mouse muscle in vivo[J]. Science, 1990, 247( 4949): 1465- 1468. |
| 11 | MARTINON F, KRISHNAN S, LENZEN G, et al.. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA[J]. Eur. J. Immunol., 1993, 23( 7): 1719- 1722. |
| 12 | SCHLAKE T, THESS A, FOTIN-MLECZEK M, et al.. Developing mRNA-vaccine technologies[J]. RNA Biol., 2012, 9( 11): 1319- 1330. |
| 13 | YOUN H, KCHUNG J. Modified mRNA as an alternative to plasmid DNA (pDNA) for transcript replacement and vaccination therapy[J]. Expert Opin. Biol. Ther., 2015, 15( 9): 1337- 1348. |
| 14 | XIAO W, WANG D. The electrochemical reduction processes of solid compounds in high temperature molten salts[J]. Chem. Soc. Rev., 2014, 43( 10): 3215- 3228. |
| 15 | KUHN A N, DIKEN M, KREITER S, et al.. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo [J]. Gene Ther., 2010, 17( 8): 961- 971. |
| 16 | RAUCH S, ROTH N, SCHWENDT K, et al.. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents[J/OL]. NPJ Vaccines, 2021, 6( 1): 57[ 2024-06-14]. . |
| 17 | VERBEKE R, LENTACKER I, DE SMEDT S C, et al.. The dawn of mRNA vaccines: the COVID-19 case[J]. J. Control. Release, 2021, 333: 511- 520. |
| 18 | BLAKNEY A K, IP S, GEALL A J. An update on self-amplifying mRNA vaccine development[J/OL]. Vaccines, 2021, 9( 2): 97[ 2024-06-14]. . |
| 19 | GREER C E, ZHOU F, LEGG H S, et al.. A chimeric alphavirus RNA replicon gene-based vaccine for human parainfluenza virus type 3 induces protective immunity against intranasal virus challenge[J]. Vaccine, 2007, 25( 3): 481- 489. |
| 20 | MOYO N, VOGEL A B, BUUS S, et al.. Efficient induction of T cells against conserved HIV-1 regions by mosaic vaccines delivered as self-amplifying mRNA[J]. Mol. Ther. Methods Clin. Dev., 2019, 12: 32- 46. |
| 21 | VOGEL A B, LAMBERT L, KINNEAR E, et al.. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses[J]. Mol. Ther., 2018, 26( 2): 446- 455. |
| 22 | AJBANI S P, VELHAL S M, KADAM R B, et al.. Immunogenicity of virus-like Semliki Forest virus replicon particles expressing Indian HIV-1C gag, env and polRT genes[J]. Immunol. Lett., 2017, 190: 221- 232. |
| 23 | KARIKÓ K, BUCKSTEIN M, NI H, et al.. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA[J]. Immunity, 2005, 23( 2): 165- 175. |
| 24 | YIN H, KANASTY R L, ELTOUKHY A A, et al.. Non-viral vectors for gene-based therapy[J]. Nat. Rev. Genet., 2014, 15( 8): 541- 555. |
| 25 | KORMANN M S D, HASENPUSCH G, ANEJA M K, et al.. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice[J]. Nat. Biotechnol., 2011, 29( 2): 154- 157. |
| 26 | MAYS L E, AMMON-TREIBER S, MOTHES B, et al.. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism[J]. J. Clin. Invest., 2013, 123( 3): 1216- 1228. |
| 27 | CHEN G L, LI X F, DAI X H, et al.. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial[J]. Lancet Microbe, 2022, 3( 3): 193- 202. |
| 28 | PARDI N, HOGAN M J, PORTER F W, et al.. mRNA vaccines-a new era in vaccinology[J]. Nat. Rev. Drug Discov., 2018, 17( 4): 261- 279. |
| 29 | SAHIN U, KARIKÓ K, TÜRECI Ö. mRNA-based therapeutics-developing a new class of drugs[J]. Nat. Rev. Drug Discov., 2014, 13: 759- 780. |
| 30 | BATISTA NAPOTNIK T, POLAJŽER T, MIKLAVČIČ D. Cell death due to electroporation-a review[J/OL]. Bioelectrochem. Amsterdam Neth., 2021, 141: 107871[ 2024-06-14]. . |
| 31 | CU Y, BRODERICK K E, BANERJEE K, et al.. Enhanced delivery and potency of self-amplifying mRNA vaccines by electroporation in situ [J]. Vaccines, 2013, 1( 3): 367- 383. |
| 32 | GAY C L, DEBENEDETTE M A, TCHEREPANOVA I Y, et al.. Immunogenicity of AGS-004 dendritic cell therapy in patients treated during acute HIV infection[J]. Aids Res. Hum. Retrov., 2018, 34( 1): 111- 122. |
| 33 | BELL G D, YANG Y, LEUNG E, et al.. mRNA transfection by a xentry-protamine cell-penetrating peptide is enhanced by TLR antagonist E6446[J/OL]. PLoS ONE, 2018, 13( 7): e 0201464[ 2024-06-14]. . |
| 34 | ALDOSARI B N, ALFAGIH I M, ALMURSHEDI A S. Lipid nanoparticles as delivery systems for RNA-based vaccines[J/OL]. Pharmaceutics, 2021, 13( 2): 206[ 2024-06-14]. . |
| 35 | RAUCH S, JASNY E, SCHMIDT K E, et al.. New vaccine technologies to combat outbreak situations[J/OL]. Front. Immunol., 2018, 9: 1963[ 2024-06-14]. . |
| 36 | CHUNG J Y, THONE M N, KWON Y J. COVID-19 vaccines: the status and perspectives in delivery points of view[J]. Adv. Drug Deliv. Rev., 2021, 170: 1- 25. |
| 37 | KOWALCZYK A, DOENER F, ZANZINGER K, et al.. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity[J]. Vaccine, 2016, 34( 33): 3882- 3893. |
| 38 | DU K, ZHENG K, CHEN Z, et al.. Unusual temperature effect on the stability of nickel anodes in molten carbonates[J]. Electrochim. Acta, 2017, 245: 410- 416. |
| 39 | CHU L, MCPHEE R, HUANG W, et al.. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine[J]. Vaccine, 2021, 39( 20): 2791- 2799. |
| 40 | LAMB Y N. BNT162b2 mRNA COVID-19 vaccine: first approval[J]. Drugs, 2021, 81( 4): 495- 501. |
| 41 | DE ALWIS R, GAN E S, CHEN S, et al.. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice[J]. Mol. Ther., 2021, 29( 6): 1970- 1983. |
| 42 | ZHANG N N, LI X F, DENG Y Q, et al.. A thermostable mRNA vaccine against COVID-19[J]. Cell, 2020, 182( 5): 1271- 1283. |
| 43 | ALAMEH M G, WEISSMAN D, PARDI N. Messenger RNA-based vaccines against infectious diseases[J]. Curr. Top. Microbiol. Immunol., 2022, 440: 111- 145. |
| 44 | FREYN A W, RAMOS DA SILVA J, ROSADO V C, et al.. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice[J]. Mol. Ther., 2020, 28( 7): 1569- 1584. |
| 45 | MCMAHON M, O'DELL G, TAN J, et al.. Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses[J/OL]. Proc. Natl. Acad. Sci. USA, 2022, 119( 45): e 2206333119[ 2024-06-14]. . |
| 46 | AREVALO C P, BOLTON M J, LE SAGE V, et al.. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes[J]. Science, 2022, 378( 6622): 899- 904. |
| 47 | PARDI N, CARREÑO J M, O'DELL G, et al.. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses[J/OL]. Nat. Commun., 2022, 13( 1): 4677[ 2024-06-14]. . |
| 48 | BAHL K, SENN J J, YUZHAKOV O, et al.. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses[J/OL]. Mol. Ther., 2022, 30( 8): 2874[ 2024-06-14]. . |
| 49 | FELDMAN R A, FUHR R, SMOLENOV I, et al.. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials[J]. Vaccine, 2019, 37( 25): 3326- 3334. |
| 50 | ALBERER M, GNAD-VOGT U, HONG H S, et al.. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial[J]. Lancet, 2017, 390( 10101): 1511- 1520. |
| 51 | ARMBRUSTER N, JASNY E, PETSCH B. Advances in RNA vaccines for preventive indications: a case study of A vaccine against rabies[J/OL]. Vaccines, 2019, 7( 4): 132[ 2024-06-14]. . |
| 52 | DAGAN N, BARDA N, KEPTEN E, et al.. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting[J]. N. Engl. J. Med., 2021, 384( 15): 1412- 1423. |
| 53 | INGLÉS TORRUELLA J, GIL SOTO R M, SABATÉ AGUILA E, et al.. Reactogenicity study of mRNA vaccines against COVID-19[J]. Arch. Prev. Riesgos Labor., 2023, 26( 2): 106- 126. |
| [1] | 常东峰, 孙召朋. mRNA药物的结构和临床应用[J]. 生物技术进展, 2024, 14(1): 1-16. |
| [2] | 蔚丹, 马云龙, 万方, 武建强. mRNA疫苗的研究及应用进展[J]. 生物技术进展, 2023, 13(4): 492-498. |
| [3] | 门佩璇, 肖宇锋, 张玢. 基于计量学方法分析数字PCR技术的临床应用现状与技术热点[J]. 生物技术进展, 2022, 12(4): 606-613. |
| [4] | 寻治铭,赵清辉,琚芳迪,何晋,姚婷婷,赵鹏翔,马雪梅,谢飞. 氢分子在临床应用中的研究进展[J]. 生物技术进展, 2019, 9(3): 217-222. |
| [5] | 何洁, 郭采平. 人转铁蛋白的分离纯化及临床应用进展[J]. 生物技术进展, 2019, 9(1): 28-34. |
| [6] | 陆腾飞,邬杨楠,裴文华,马月辉,关伟军. 内皮祖细胞的生物学特性及其应用[J]. 生物技术进展, 2017, 7(4): 266-271. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部
