


生物技术进展 ›› 2022, Vol. 12 ›› Issue (4): 481-489.DOI: 10.19586/j.2095-2341.2022.0115
• 氢生物医学专题 • 下一篇
收稿日期:2022-06-30
接受日期:2022-07-03
出版日期:2022-07-25
发布日期:2022-08-10
通讯作者:
谢飞,马雪梅
作者简介:宋怡菲 E-mail:S202165052@emails.bjut.edu.cn
基金资助:
Yifei SONG( ), Fei XIE(
), Fei XIE( ), Chen MA, Xuemei MA(
), Chen MA, Xuemei MA( )
)
Received:2022-06-30
Accepted:2022-07-03
Online:2022-07-25
Published:2022-08-10
Contact:
Fei XIE,Xuemei MA
摘要:
氢化酶作为一种可催化氢气氧化与质子还原的金属酶,在生物体的氢代谢过程中发挥着关键作用。已有研究表明,氢气干预可对植物的生长发育和抗逆性产生积极影响,同时一些高等植物的内源性产氢现象也已得到证实,然而关于催化内源性产氢的氢化酶目前了解较少。虽然已有多项研究表明,叶绿体可能是高等植物产氢的关键部位,但是鉴于多种植物在种子萌发时仍然可以产氢,而种子萌发过程中叶绿体还没有生成,加上氢化酶在进化上与线粒体复合物Ⅰ具有同源性,在对氢化酶研究现状进行概述的基础上,提出了高等植物线粒体具有氢化酶活性的猜想,并总结了线粒体存在氢化酶活性的初步实验证据,以期为后续线粒体与氢化酶的关系研究提供参考依据。
中图分类号:
宋怡菲, 谢飞, 马晨, 马雪梅. 高等植物氢化酶活性研究进展[J]. 生物技术进展, 2022, 12(4): 481-489.
Yifei SONG, Fei XIE, Chen MA, Xuemei MA. Research Progress on Hydrogenase Activity in Higher Plants[J]. Current Biotechnology, 2022, 12(4): 481-489.
| 植物种类 | 产氢/耗氢现象 | 发生部位/过程 | 参考文献 |
|---|---|---|---|
| 冬黑麦、萝卜、豌豆、番茄 | 产氢 | 种子萌发过程中 | [ |
| 豌豆 | 产氢+耗氢 | 离体叶绿体 | [ |
| 大麦 | 产氢 | 厌氧培养的大麦幼苗 | [ |
| 豌豆、菠菜 | 产氢 | 离体叶绿体 | [ |
| 水稻 | 产氢 | 水稻幼苗 | [ |
| 拟南芥 | 产氢 | 拟南芥幼苗 | [ |
| 苜蓿 | 产氢 | 苜蓿幼苗 | [ |
| 猕猴桃 | 产氢 | 新鲜果肉组织 | [ |
| 番茄 | 产氢 | 新鲜果肉组织 | [ |
| 洋桔梗 | 产氢 | 新鲜花茎 | [ |
| 绿豆、辣椒 | 产氢+耗氢 | 植物幼苗中纯化出的质膜 | [ |
表1 不同高等植物中的氢代谢现象
Table 1 Hydrogen metabolism in different higher plants
| 植物种类 | 产氢/耗氢现象 | 发生部位/过程 | 参考文献 |
|---|---|---|---|
| 冬黑麦、萝卜、豌豆、番茄 | 产氢 | 种子萌发过程中 | [ |
| 豌豆 | 产氢+耗氢 | 离体叶绿体 | [ |
| 大麦 | 产氢 | 厌氧培养的大麦幼苗 | [ |
| 豌豆、菠菜 | 产氢 | 离体叶绿体 | [ |
| 水稻 | 产氢 | 水稻幼苗 | [ |
| 拟南芥 | 产氢 | 拟南芥幼苗 | [ |
| 苜蓿 | 产氢 | 苜蓿幼苗 | [ |
| 猕猴桃 | 产氢 | 新鲜果肉组织 | [ |
| 番茄 | 产氢 | 新鲜果肉组织 | [ |
| 洋桔梗 | 产氢 | 新鲜花茎 | [ |
| 绿豆、辣椒 | 产氢+耗氢 | 植物幼苗中纯化出的质膜 | [ |
| 研究对象 | 作用领域 | 作用效果 | 参考文献 |
|---|---|---|---|
| 拟南芥 | 参与植物抗生物与非生物胁迫 | 提高拟南芥耐盐性 | [ |
| 紫花苜蓿 | 参与植物抗生物与非生物胁迫 | 增强苜蓿对百草枯诱导的氧化胁迫耐受性 | [ |
| 参与植物抗生物与非生物胁迫 | 增强苜蓿的镉耐受性 | [ | |
| 参与植物抗生物与非生物胁迫 | 缓解苜蓿的渗透胁迫 | [ | |
| 水稻 | 参与植物抗生物与非生物胁迫 | 缓解水稻种子萌发过程中的盐胁迫 | [ |
| 黄瓜 | 参与植物抗生物与非生物胁迫 | 缓解黄瓜植株所受干旱胁迫 | [ |
| 猕猴桃 | 改善植物品质与保鲜 | 延缓猕猴桃采后成熟和衰老 | [ |
| 洋桔梗 | 改善植物品质与保鲜 | 延缓洋桔梗鲜切花花瓣衰老 | [ |
| 番茄 | 改善植物品质与保鲜 | 抑制番茄果实贮藏中亚硝酸盐的积累 | [ |
| 小苍兰 | 改善植物品质与保鲜 | 增加叶片、花茎长度与花朵直径 | [ |
| 番茄 | 参与调控植物生长发育 | 参与诱导番茄幼苗的侧根形成 | [ |
| 黑麦 | 参与调控植物生长发育 | 促进黑麦种子的萌发 | [ |
| 万寿菊 | 参与调控植物生长发育 | 调控万寿菊的不定根发育 | [ |
表2 氢分子对高等植物生理过程的调控
Table 2 Regulation of some physiological processes in higher plants by hydrogen molecules
| 研究对象 | 作用领域 | 作用效果 | 参考文献 |
|---|---|---|---|
| 拟南芥 | 参与植物抗生物与非生物胁迫 | 提高拟南芥耐盐性 | [ |
| 紫花苜蓿 | 参与植物抗生物与非生物胁迫 | 增强苜蓿对百草枯诱导的氧化胁迫耐受性 | [ |
| 参与植物抗生物与非生物胁迫 | 增强苜蓿的镉耐受性 | [ | |
| 参与植物抗生物与非生物胁迫 | 缓解苜蓿的渗透胁迫 | [ | |
| 水稻 | 参与植物抗生物与非生物胁迫 | 缓解水稻种子萌发过程中的盐胁迫 | [ |
| 黄瓜 | 参与植物抗生物与非生物胁迫 | 缓解黄瓜植株所受干旱胁迫 | [ |
| 猕猴桃 | 改善植物品质与保鲜 | 延缓猕猴桃采后成熟和衰老 | [ |
| 洋桔梗 | 改善植物品质与保鲜 | 延缓洋桔梗鲜切花花瓣衰老 | [ |
| 番茄 | 改善植物品质与保鲜 | 抑制番茄果实贮藏中亚硝酸盐的积累 | [ |
| 小苍兰 | 改善植物品质与保鲜 | 增加叶片、花茎长度与花朵直径 | [ |
| 番茄 | 参与调控植物生长发育 | 参与诱导番茄幼苗的侧根形成 | [ |
| 黑麦 | 参与调控植物生长发育 | 促进黑麦种子的萌发 | [ |
| 万寿菊 | 参与调控植物生长发育 | 调控万寿菊的不定根发育 | [ |
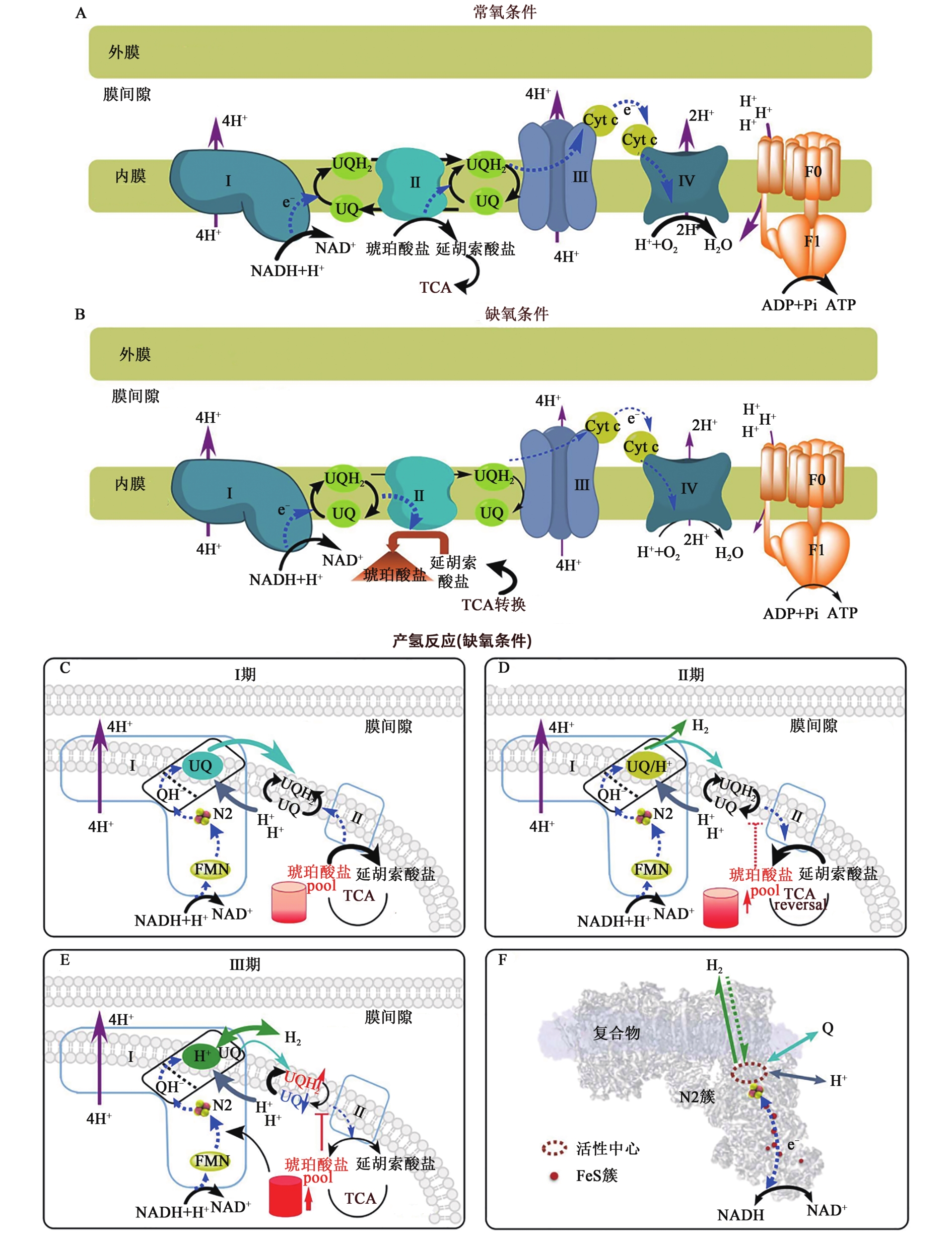
图1 线粒体产氢机制示意图[50]A:常氧条件下的电子传递链示意图;B:缺氧条件下的电子传递链示意图;C:产氢Ⅰ期的正常TCA循环;D:琥珀酸在Ⅱ期积累,开始产氢;E:琥珀酸在Ⅲ期进一步积累,大量产氢;F:位于复合物Ⅰ的N2位点附近的产氢活性中心。UQ—氧化型泛醌; UQH2—还原型泛醌;QH-—半还原型泛醌;Cyt c—细胞色素c;TCA—三羧酸循环;NADH—还原型烟酰胺腺嘌呤二核苷酸;NAD+—烟酰胺腺嘌呤二核苷酸;ADP—二磷酸腺苷;ATP—三磷酸腺苷;FMN—黄素单核苷酸。
Fig. 1 The schematic diagram of H2 evolution mechanism in mitochondria[50]
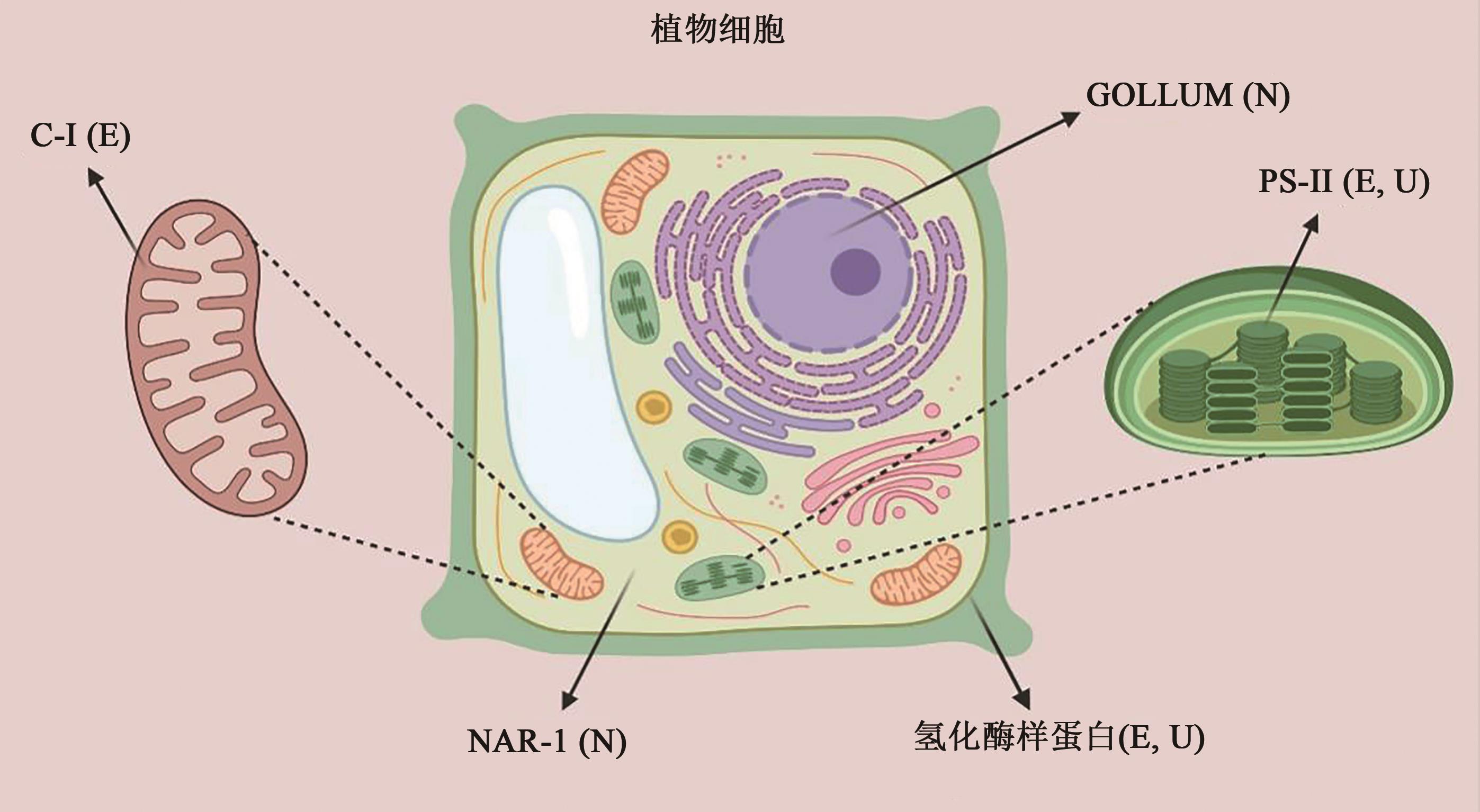
图2 高等植物潜在氢化酶的亚细胞定位及其催化活性注:C-Ⅰ—线粒体复合物Ⅰ;PS-Ⅱ—叶绿体光系统Ⅱ;E(evolution)—产氢;U(uptake)—吸氢;N(not available)—产氢/吸氢活性未知;NAR-1—拟南芥中的[Fe-Fe]氢化酶样蛋白;GOLLUM—苜蓿中的[Fe-Fe]氢化酶样蛋白。
Fig. 2 The subcellular localization and catalytic activity of the potential hydrogenase in higher pants
| 1 | TORRES V, BALLESTEROS A, FERNÁNDEZ V M. Expression of hydrogenase activity in barley (Hordeum vulgare L.) after anaerobic stress[J]. Arch. Biochem. Biophys., 1986, 245(1): 174-178. |
| 2 | XIE Y, MAO Y, ZHANG W, et al.. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis [J]. Plant Physiol., 2014, 165(2): 759-773. |
| 3 | JIN Q, ZHU K, CUI W, et al.. Hydrogen-modulated stomatal sensitivity to abscisic acid and drought tolerance via the regulation of apoplastic pH in Medicago sativa [J]. J. Plant Grow. Regul., 2016, 35(2): 565-573. |
| 4 | MAL'TSEV S V, ALLAKHVERDIEV S I, KLIMOV V V, et al.. Hydrogen evolution by subchloroplast preparations of photosystem II from pea and spinach[J]. FEBS Lett., 1988, 240(1-2): 1-5. |
| 5 | XIE X Y, MAO Y, LAI D, et al.. H2 enhances Arabidopsis salt tolerance by manipulating ZAT1012-mediated antioxidant defence and controlling sodium exclusion[J]. PLoS ONE, 2012, 7(11):1-11. |
| 6 | JIN Q, ZHU K, CUI W, et al.. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat‐induced oxidative stress via the modulation of heme oxygenase‐1 signaling system[J]. Plant Cell Environ., 2013, 36(5): 956-969. |
| 7 | XU S, ZHU S, JIANG Y, et al.. Hydrogen-rich water alleviates salt stress in rice during seed germination[J]. Plant Soil, 2013, 370(1): 47-57. |
| 8 | CUI W, YAO P, PAN J, et al.. Transcriptome analysis reveals insight into molecular hydrogen-induced cadmium tolerance in alfalfa: the prominent role of sulfur and (homo) glutathione metabolism[J]. BMC Plant Biol., 2020, 20(1): 1-19. |
| 9 | SU J, ZHANG Y, NIE Y, et al.. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings[J]. Environ. Exp. Bot., 2018, 147(1):249-260. |
| 10 | HU H, LI P, WANG Y, et al.. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit[J]. Food Chem., 2014, 156(1):100-109. |
| 11 | HU H, ZHAO S, LI P, et al.. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis[J]. Posthar. Biol. Technol., 2018, 135(1):123-130. |
| 12 | SU J, NIE Y, ZHAO G, et al.. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers[J]. Posthar. Biol. Technol., 2019, 147(1):148-155. |
| 13 | ZHANG Y, ZHAO G, CHENG P, et al.. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas[J]. Int. J. Food Propert., 2019, 22(1): 1425-1438. |
| 14 | EMBLEY M, GIEZEN M, HORNER D S, et al.. Mitochondria and hydrogenosomes are two forms of the same fundamental organelle[J]. Philos. Trans. Royal Soc. London B Biol. Sci., 2003, 358(1429): 191-203. |
| 15 | EFREMOV R G, SAZANOV L A. The coupling mechanism of respiratory complex I—a structural and evolutionary perspective[J]. Biochim. Biophys. Acta Bioenerg., 2012, 1817(10): 1785-1795. |
| 16 | LUBITZ W, OGATA H, RUDIGER O, et al.. Hydrogenases[J]. Chem. Rev., 2014, 114(8): 4081-4148. |
| 17 | DE LACEY A L, FERNANDEZ V M, ROUSSET M, et al.. Activation and inactivation of hydrogenase function and the catalytic cycle: Spectro electrochemical studies[J]. Chem. Rev., 2007, 107(10): 4304-4330. |
| 18 | AGUILERA-CAMPOS K I, STAIRS C W. Hydrogen metabolism: a eukaryote taps into the electron sink[J]. Curr. Biol., 2022, 32(1): 49-51. |
| 19 | STEPHENSON M, STICKLAND L H. Hydrogenase: A bacterial enzyme activating molecular hydrogen: the properties of the enzyme[J]. Biochem. J., 1931, 25(1):205-214. |
| 20 | CHEN J S, MORTENSON L E. Purification and properties of hydrogenase from Clostridium pasteurianum W5[J]. Biochim. Biophys. Acta Protein Struct., 1974, 371(2): 283-298. |
| 21 | TAMAGNINI P, LEITO E, OLIVEIRA P, et al.. Cyanobacterial hydrogenases: diversity, regulation and applications[J]. FEMS Microbiol. Rev., 2007, 31(6):692-720. |
| 22 | HAPPE T, NABER J D. Isolation, characterization and N‐terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii [J]. Eur. J. Biochem., 1993, 214(2): 475-481. |
| 23 | HAPPE T, MOSLER B, NABER J D. Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii [J]. Eur. J. Biochem., 1994, 222(3): 769-774. |
| 24 | HEMSCHEMEIER A, FOUCHARD S, COURNAC L, et al.. Hydrogen production by Chlamydomonas reinhardtii: An elaborate interplay of electron sources and sinks[J]. Planta, 2008, 227(2): 397-407. |
| 25 | FORESTIER M, KING P, ZHANG L, et al.. Expression of two [Fe]‐hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions[J]. Eur. J. Biochem., 2003, 270(13): 2750-2758. |
| 26 | FLORIN L, TSOKOGLOU A, HAPPE T. A novel type of iron hydrogenase in the green Alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain[J]. J. Biol. Chem., 2001, 276(9): 6125-6132. |
| 27 | COURNAC L, GUEDENEY G, PELTIER G, et al.. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type Ⅰ NADPH-dehydrogenase complex[J]. J. Bacteriol., 2004, 186(6): 1737-1746. |
| 28 | NÝVLTOVÁ E, ŠUTÁK R, HARANT K, et al.. NIF-type iron-sulfur cluster assembly system is duplicated and distributed in the mitochondria and cytosol of Mastigamoeba balamuthi [J]. Proc. Natl. Acad. Sci. USA, 2013, 110(18): 7371-7376. |
| 29 | BOICHENKO E A. Evolution of hydrogen by isolated chloroplasts[J]. Compt. Rend. Acad. Sci. USSR, 1946, 52(1):521-524. |
| 30 | GIUMARRO C, SIEGEL S M. Hydrogen metabolism in higher plants[J]. Plant Physiol., 1964, 39(3): 303-306. |
| 31 | MAL'TSEV S V, KRASNOVSKII A A. Production and consumption of molecular hydrogen by isolated chloroplasts of higher plants[J]. Soviet Plant Physiol., 1982, 29(5): 951-958. |
| 32 | ZENG J, ZHANG M, SUN X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants[J]. PLoS ONE, 2013, 8(8): 1-10. |
| 33 | ZHANG X, XIE F, ZHANG Z, et al.. Hydrogen evolution and absorption phenomena in the plasma membrane of Vigna radiata and Capsicum annuum [J]. J. Plant Growth Regul., 2022, 5(1):1-11. |
| 34 | 沈文飚, 苏久厂, 孙学军.氢气植物学效应的研究进展[J].南京农业大学学报, 2018, 41(3):392-401. |
| 35 | ZULFIQAR F, RUSSELLG, HANCOCK J T. Molecular hydrogen in agriculture[J]. Planta, 2021, 254(3): 1-14. |
| 36 | CHEN Y, WANG M, HU L, et al.. Carbon monoxide is involved in hydrogen gas-induced adventitious root development in cucumber under simulated drought stress[J].Front. Plant Sci., 2017, 8(128): 1-16. |
| 37 | 宋韵琼,沙米拉·太来提,杜红梅.富氢水处理对小苍兰生长发育的影响[J].上海交通大学学报(农业科学版),2016,34(3):55-61, 96. |
| 38 | CAO Z, DUAN X, YAO P, et al.. Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis[J]. Int. J. Mol. Sci., 2017, 18(2084):1-15. |
| 39 | RENWICK G M, GIUMARRO C, SIEGEL S M. Hydrogen metabolism in higher plants[J]. Plant Physiol., 1964, 39(3):303-306. |
| 40 | ZHU Y, LIAO W. The metabolic constituent and rooting-related enzymes responses of marigold explants to hydrogen gas during adventitious root development[J]. Theor. Exp. Plant Physiol., 2017, 29(2): 77-85. |
| 41 | NAKAMURA M, BUZAS D M, KATO A, et al.. The role of Arabidopsis thaliana NAR 1, a cytosolic iron-sulfur cluster assembly component, in gametophytic gene expression and oxidative stress responses in vegetative tissue[J]. New Phytol., 2013, 199(4): 925-935. |
| 42 | MONDY S, LENGLET A, COSSON V, et al.. GOLLUM [Fe-Fe]‐hydrogenase‐like proteins are essential for plant development in normoxic conditions and modulate energy metabolism[J]. Plant Cell Environ., 2014, 37(1): 54-69. |
| 43 | EMBLEY T M, MARTIN W. Eukaryotic evolution, changes and challenges[J]. Nature, 2006, 440(7084): 623-630. |
| 44 | MÜER M Ó. The hydrogenosome[J]. Microbiology, 1993, 139(12): 2879-2889. |
| 45 | BOXMA B, DE GRAAF R M, STAAY G W M, et al.. An anaerobic mitochondrion that produces hydrogen[J]. Nature, 2005, 434(7029): 74-79. |
| 46 | TIELENS A G M, ROTTE C, HELLEMOND J J, et al.. Mitochondria as we don't know them[J]. Trends Biochem. Sci., 2002, 27(11): 564-572. |
| 47 | BÖHM R, SAUTER M, BÖCK A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenylase components[J]. Mol. Microbiol., 1990, 4(2): 231-243. |
| 48 | MOPARTHI V K, HÄGERHÄLL C. The evolution of respiratory chain complex I from a smaller last common ancestor consisting of 11 protein subunits[J]. J. Mol. Evol., 2011, 72(5): 484-497. |
| 49 | YU H, WU C H, SCHUT G J, et al.. Structure of an ancient respiratory system[J]. Cell, 2018, 173(7): 1636-1649. |
| 50 | ZHANG X, ZHANG Z, WEI Y, et al.. Mitochondria in higher plants possess H2 evolving activity which is closely related to complex I[J/OL]. arXiv,2001,02132: 2020[2022-07-03].. |
| 51 | 马雪梅,张鑫,谢飞,等.氢气生物学作用的生物酶基础[J].生物技术进展,2020,10(1):15-22. |
| [1] | 刘梓嘉, 姜雪, 仪杨, 王濛, 马晨, 宋怡菲, 谢飞. 氢气与肠道菌群的关系研究进展[J]. 生物技术进展, 2022, 12(6): 847-852. |
| [2] | 张晓, 李才华, 王婧, 张兰兰, 牟晓雨, 王昕玥, 甘刘美, 周鹏展, 张锐. 基因加倍对棉花线粒体atpA基因RNA编辑率的影响[J]. 生物技术进展, 2022, 12(5): 737-745. |
| [3] | 侯凯耀, 张二飞, 郑李娜, 陈红光, 谢克亮. 富氢液对脓毒症小鼠心肌细胞线粒体自噬的影响[J]. 生物技术进展, 2022, 12(4): 497-502. |
| [4] | 马雪梅,张鑫,谢飞,赵鹏翔,张昭,仪杨,张晓康,马胜男,李秦剑,吕宝北,刘梦昱,YAO Mawulikplimi Adzavon,孙学军,李英贤. 氢气生物学作用的生物酶基础[J]. 生物技术进展, 2020, 10(1): 15-22. |
| [5] | 林晶晶,杨宇丰. 线粒体自噬的调控机制及其在相关疾病中的作用[J]. 生物技术进展, 2019, 9(5): 467-475. |
| [6] | 王裴林,周利利,梁成真,孟志刚,郭三堆,张锐. 棉花线粒体基因cRT-PCR改良及其在寻找CMS相关基因中的应用[J]. 生物技术进展, 2019, 9(3): 303-308. |
| [7] | 孟晓庆,侯智霞,张兰. 高等植物成花转变分子调控机制研究进展生物技术进展[J]. 生物技术进展, 2013, 3(1): 1-6. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部