


生物技术进展 ›› 2024, Vol. 14 ›› Issue (5): 785-792.DOI: 10.19586/j.2095-2341.2024.0023
• 进展评述 • 上一篇
收稿日期:2024-02-20
接受日期:2024-06-04
出版日期:2024-09-25
发布日期:2024-10-22
通讯作者:
于宁
作者简介:唐金格 E-mail: tangjinge6@163.com;
基金资助:
Jinge TANG1,2( ), Wei REN2, Qingqing JIANG2, Ning YU1(
), Wei REN2, Qingqing JIANG2, Ning YU1( )
)
Received:2024-02-20
Accepted:2024-06-04
Online:2024-09-25
Published:2024-10-22
Contact:
Ning YU
摘要:
稳定同位素因具有无放射性、稳定性好等特点,在医药、食品安全、生态环境等领域应用广泛。氘作为氢元素的一种稳定同位素,氘元素标记法具有简单、快捷、价格低等优点。研究发现,氘同位素标记在机体代谢机制、疾病预防及治疗靶点、药理学等生命科学领域具有巨大的应用潜力。综述从氘标记化合物的合成方法、检测技术、氘及其化合物在生物医药研究中的应用等方面进行总结,并对未来发展进行了展望,以期为相关领域的研究与应用提供参考。
中图分类号:
唐金格, 任巍, 蒋晴晴, 于宁. 氘及其标记化合物在生物医药研究中的应用进展[J]. 生物技术进展, 2024, 14(5): 785-792.
Jinge TANG, Wei REN, Qingqing JIANG, Ning YU. Progress in the Application of Deuterium and its Labeled Compounds in Biomedical Research[J]. Current Biotechnology, 2024, 14(5): 785-792.
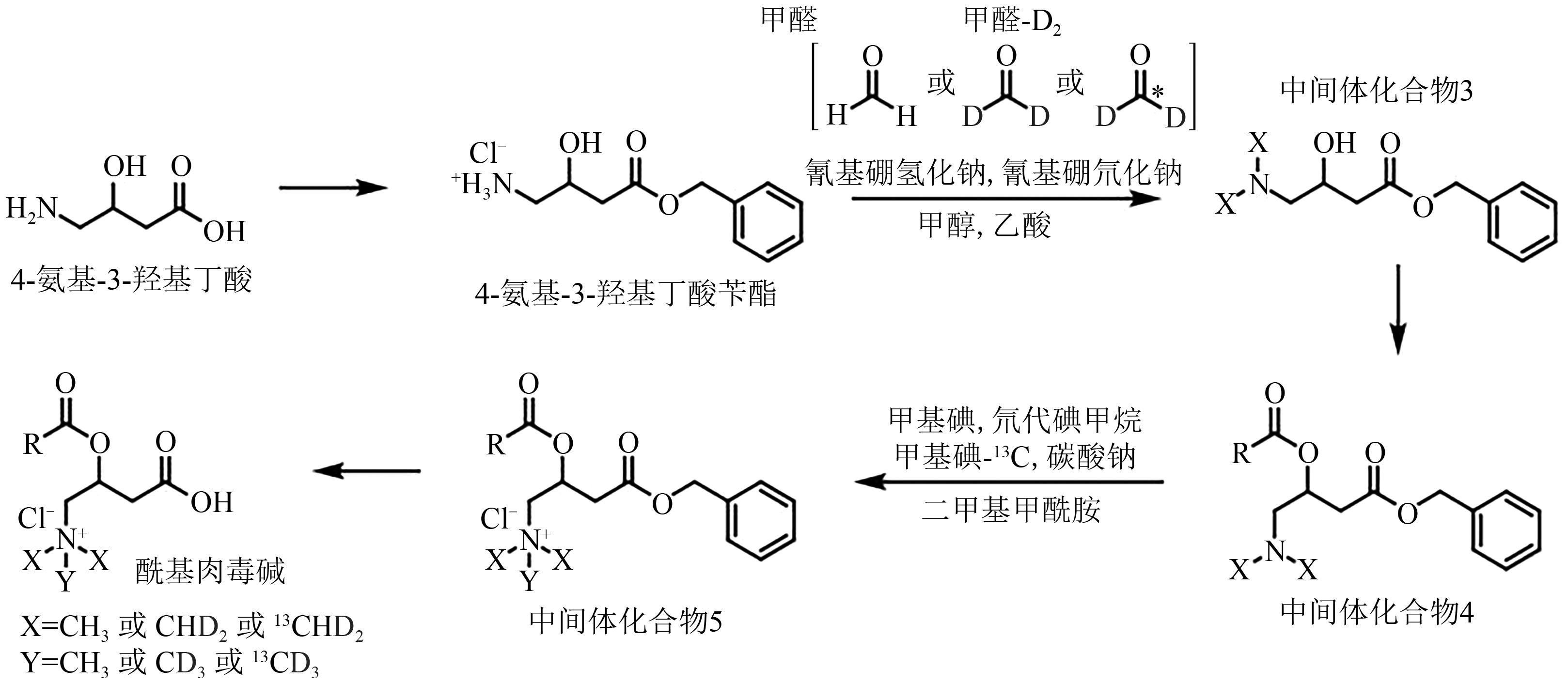
图4 酰基肉毒碱的可调谐合成[17]注:NaCNBH3—氰基硼氢化钠;NaCNBD3—氰基硼氘化钠;MeOH—甲醇;AcOH—乙酸;DMF—二甲基甲酰胺;CH3I—甲基碘;CD3I—氘代碘甲烷;13CD3I—甲基碘-13C;Na2CO3—碳酸钠。
Fig. 4 Tuneable synthesis of acylcarnitines[17]
| 1 | VOGES R, HEYS J, MOENIUS T. Preparation of tritium‐labelled compounds by isotope exchange reactions[M]. German:Wiley, 2009, 47-107. |
| 2 | ATZRODT J, DERDAU V, KERR W J, et al.. Deuterium- and tritium-labelled compounds: applications in the life sciences[J]. Angew. Chem. Int. Ed., 2018, 57(7): 1758-1784. |
| 3 | YANG H, HESK D. Base metal-catalyzed hydrogen isotope exchange[J]. J. Label. Compd. Radiopharm., 2020, 63(6): 296-307. |
| 4 | DI GIUSEPPE A, CASTARLENAS R, ORO L A. Mechanistic considerations on catalytic H/D exchange mediated by organometallic transition metal complexes[J]. C. R. Chim., 2015, 18(7): 713-741. |
| 5 | LIU M, CHEN X, CHEN T, et al.. A facile and general acid-catalyzed deuteration at methyl groups of N-heteroarylmethanes[J]. Org. Biomol. Chem., 2017, 15(12): 2507-2511. |
| 6 | SAJIKI H, SAWAMA Y, MONGUCHI Y. Efficient H-D exchange reactions using heterogeneous platinum-group metal on carbon-H2-D2O system[J]. Synlett, 2012, 23(7): 959-972. |
| 7 | CHATTERJEE B, KRISHNAKUMAR V, GUNANATHAN C. Selective α‐deuteration of amines and amino acids using D2O[J]. Org. Lett., 2016, 18(22): 5892‐5895. |
| 8 | LEGROS F, FERNANDEZ-RODRIGUEZ P, MISHRA A, et al.. Photoredox-mediated hydrogen isotope exchange reactions of amino-acids, peptides, and peptide-derived drugs[J]. Chemistry, 26(56): 12738-12742. |
| 9 | LOH Y Y, NAGAO K, HOOVER A J, et al.. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds[J]. Science, 2017, 358(6367): 1182-1187. |
| 10 | KOPF S, BOURRIQUEN F, LI W, et al.. Recent developments for the deuterium and tritium labeling of organic molecules[J]. Chem. Rev., 2022, 122(6): 6634-6718. |
| 11 | LIU C, CHEN Z, SU C, et al.. Controllable deuteration of halogenated compounds by photocatalytic D2O splitting[J/OL]. Nat. Commun., 2018, 9(1): 80[2024-07-22]. . |
| 12 | GOU X Y, LI Y, WANG X G, et al.. Ruthenium-catalyzed ortho-selective CAr-H amination of heteroaryl arenes with di-tert-butyldiaziridinone[J]. Chem. Commun., 2019, 55(38): 5487-5490. |
| 13 | RUIZ-CASTANEDA M, CARRIÓN M C, SANTOS L, et al.. A biphasic medium slows down the transfer hydrogenation and allows a selective catalytic deuterium labeling of amines from imines mediated by a Ru-H/D+ exchange in D2O[J]. ChemCatChem, 2018,10(23): 5541-5550. |
| 14 | COOK A, PRAKASH S, ZHENG Y L, et al.. Exhaustive reduction of esters enabled by nickel catalysis[J]. J. Am. Chem. Soc., 2020, 142(18): 8109-8115. |
| 15 | FENG K, QUEVEDO R E, KOHRT J T, et al.. Late-stage oxidative C(sp3)-H methylation[J]. Nature, 2020, 580(7805): 621-627. |
| 16 | SKLYARUK J, BORGHS J C, EL-SEPELGY O, et al.. Catalytic C-1 alkylation with methanol and isotope-labeled methanol[J]. Angew. Chem. Int. Edit., 2019, 58(3): 775-779. |
| 17 | DAI X, LV C, SUN J, et al.. A facile synthesis of isotope labeled acylcarnitines[J]. J. Label. Compd. Radiopharm., 2021, 64(5): 217-224. |
| 18 | HESK D, KOHARSKI D, MCNAMARA P, et al.. Synthesis of 3H, 13C2, 2H4 14C-SCH 430765 and 35S-SCH 500946, potent and selective inhibitors of the NPY5 receptor[J]. J. Label. Compd. Radiopharm., 2018, 61(7): 533-539. |
| 19 | WILKINSON D J, BROOK M S, SMITH K, et al.. Stable isotope tracers and exercise physiology: past, present and future[J]. J. Physiol., 2017, 595(9): 2873-2882. |
| 20 | WEIS D. Hydrogen exchange mass spectrometry of proteins: fundamentals, methods, and applications[M]. New York: John Wiley&Sons, 2016. |
| 21 | JAMES E I, MURPHREE T A, VORAUER C, et al.. Advances in hydrogen/deuterium exchange mass spectrometry and the pursuit of challenging biological systems[J]. Chem. Rev., 2022, 122(8): 7562-7623. |
| 22 | DENG B, LENTO C, WILSON D J. Hydrogen deuterium exchange mass spectrometry in biopharmaceutical discovery and development-a review[J]. Anal. Chim. Acta, 2016, 940: 8-20. |
| 23 | WALLS A C, FIALA B, SCHÄFER A, et al.. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2[J]. Cell, 2020, 183(5): 1367-1382. |
| 24 | PANTAZATOS D, KIM J S, KLOCK H E, et al.. Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS[J]. Proc. Natl. Acad. Sci. USA, 2004, 101(3): 751-756. |
| 25 | MASSON G R, BURKE J E, AHN N G, et al.. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments[J]. Nat. Meth., 2019, 16(7): 595-602. |
| 26 | IACOB R E, KRYSTEK S R, HUANG R Y, et al.. Hydrogen/deuterium exchange mass spectrometry applied to IL-23 interaction characteristics: potential impact for therapeutics[J]. Expert Rev. Proteom., 2015, 12(2): 159-169. |
| 27 | POLVOY I, QIN H, FLAVELL R R, et al.. Deuterium metabolic imaging-rediscovery of a spectroscopic tool[J/OL]. Metabolites, 2021, 11(9): 570[2024-07-22]. . |
| 28 | KAGGIE J D, KHAN A S, MATYS T, et al.. Deuterium metabolic imaging and hyperpolarized 13C-MRI of the normal human brain at clinical field strength reveals differential cerebral metabolism[J/OL]. NeuroImage, 2022, 257: 119284[2024-07-22]. . |
| 29 | MAJZNER K, TOTT S, ROUSSILLE L, et al.. Uptake of fatty acids by a single endothelial cell investigated by Raman spectroscopy supported by AFM[J]. Analyst, 2018, 143(4): 970-980. |
| 30 | HARTMANN B, MÜLLER M, SEYLER L, et al.. Feasibility of deuterium magnetic resonance spectroscopy of 3-O-methylglucose at 7 Tesla[J/OL]. PLoS One, 2021, 16(6): e0252935[2024-07-22]. . |
| 31 | FLOCKE V, TEMME S, BOUVAIN P, et al.. Noninvasive assessment of metabolic turnover during inflammation by in vivo deuterium magnetic resonance spectroscopy[J/OL]. Front. Immunol., 2023, 14: 1258027[2024-07-22]. . |
| 32 | HARRIS J J, JOLIVET R, ATTWELL D. Synaptic energy use and supply[J]. Neuron, 2012, 75(5): 762-777. |
| 33 | LU M, ZHU X H, ZHANG Y, et al.. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy[J]. J. Cereb. Blood Flow Metab., 2017, 37(11): 3518-3530. |
| 34 | DE FEYTER H M, BEHAR K L, CORBIN Z A, et al.. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo [J/OL]. Sci. Adv., 2018, 4(8): eaat7314[2024-07-22]. . |
| 35 | WADA Y, SATO Y, MIYAZAKI K, et al.. The reduced/oxidized state of plasma albumin is modulated by dietary protein intake partly via albumin synthesis rate in rats[J]. Nutr. Res., 2017, 37: 46-57. |
| 36 | 田颖,孟婵芳,时明慧,等.重水标记法测定大鼠血浆清蛋白合成动力的研究[J].肠外与肠内营养,2018,25(1):52-55+61. |
| TIAN Y, MENG C F, SHI M H, et al.. Kinetic parameters of plasma albumin synthesis by deuterated water[J]. Parenteral & Enteral Nutri.,2018, 25(1): 52-55+61. | |
| 37 | LARA R, SECKL M J, PARDO O E. The p90 RSK family members: common functions and isoform specificity[J]. Cancer Res., 2013, 73(17): 5301-5308. |
| 38 | CHRYSOSTOMOU S, ROY R, PRISCHI F, et al.. Repurposed floxacins targeting RSK4 prevent chemoresistance and metastasis in lung and bladder cancer[J/OL]. Sci. Transl. Med., 2021, 13(602): eaba4627[2024-07-22]. . |
| 39 | WOJCIK J, HANTSCHEL O, GREBIEN F, et al.. A potent and highly specific FN3 monobody inhibitor of the Abl SH2 domain[J]. Nat. Struct. Mol. Biol., 2010, 17(4): 519-527. |
| 40 | LANKFORD C S, FRUCHT D M. A unique role for IL-23 in promoting cellular immunity[J]. J. Leukoc. Biol., 2003, 73(1): 49-56. |
| 41 | 赵鹏翔,谢飞,刘梦昱,等.氢气生物医学研究进展[J].生物技术进展,2021,11(4):503-517. |
| ZHAO P X, XIE F, LIU M Y, et al.. Research progress in hydrogen biomedical science[J]. Curr. Biotechnol., 2021, 11(4): 503-517. | |
| 42 | HYSPLER R, TICHA A, SCHIERBEEK H, et al.. The evaluation and quantitation of dihydrogen metabolism using deuterium isotope in rats[J/OL]. PLoS ONE, 2015, 10(6): e0130687[2024-07-22]. . |
| 43 | DI MARTINO R M C, MAXWELL B D, PIRALI T. Deuterium in drug discovery: progress, opportunities and challenges[J]. Nat. Rev. Drug Discov., 2023, 22(7): 562-584. |
| 44 | SCHMIDT C. First deuterated drug approved[J]. Nat. Biotechnol., 2017, 35(6): 493-494. |
| 45 | SCOTT-STEVENS P, ATACK J R, SOHAL B, et al.. Rodent pharmacokinetics and receptor occupancy of the GABAA receptor subtype selective benzodiazepine site ligand L-838417[J]. Biopharm. Drug Dispos., 2005, 26(1): 13-20. |
| 46 | BRAMAN V, LIU J F, HARBESON S, et al.. Preliminary clinical outcomes for CTP-354, a novel subtype-selective GABA(A) modulator[J]. Ann. Neurol., 2014, 76(S18): 104-104. |
| 47 | HARBESON S L, MORGAN A J, LIU J F, et al.. Altering metabolic profiles of drugs by precision deuteration 2: discovery of a deuterated analog of ivacaftor with differentiated pharmacokinetics for clinical development[J]. J. Pharmacol. Exp. Ther., 2017, 362(2): 359-367. |
| 48 | PIRALI T, SERAFINI M, CARGNIN S, et al.. Applications of deuterium in medicinal chemistry[J]. J. Med. Chem., 2019, 62(11): 5276-5297. |
| 49 | CALINSKI D M, ZHANG H, LUDEMAN S, et al.. Hydroxylation and N-dechloroethylation of Ifosfamide and deuterated ifosfamide by the human cytochrome P450s and their commonly occurring polymorphisms[J]. Drug Metab. Dispos. Biol. Fate Chem., 2015, 43(7): 1084-1090. |
| 50 | FRAM D M, ALMENOFF J S, DUMOUCHEL W. Empirical Bayesian data mining for discovering patterns in post-marketing drug safety[C]//The Ninth ACM SIGKDD International Conference, ACM, 2003. |
| 51 | UTTAMSINGH V, GALLEGOS R, LIU J F, et al.. Altering metabolic profiles of drugs by precision deuteration: reducing mechanism-based inhibition of CYP2D6 by paroxetine[J]. J. Pharmacol. Exp. Ther., 2015, 354(1): 43-54. |
| 52 | RODRIGUEZ-VIEITEZ E, CARTER S F, CHIOTIS K, et al.. Comparison of early-phase 11C-deuterium-l-deprenyl and 11C-pittsburgh compound B PET for assessing brain perfusion in alzheimer disease[J]. J. Nucl. Med., 2016, 57(7): 1071-1077. |
| 53 | FOWLER J S, WANG G J, LOGAN J, et al.. Selective reduction of radiotracer trapping by deuterium substitution: comparison of carbon-11-L-deprenyl and carbon-11-deprenyl-D2 for MAO B mapping[J]. J. Nucl. Med., 1995, 36(7): 1255-1262. |
| 54 | DEGRADO T R, COLEMAN R E, WANG S, et al.. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer[J]. Cancer Res., 2001, 61(1): 110-117. |
| 55 | BANSAL A, WANG S, HARA T, et al.. Biodisposition and metabolism of [(18)F] fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats[J]. Eur. J. Nucl. Med. Mol., 2008, 35(6): 1192-1203. |
| [1] | 师帅, 赵迎春, 林瑞红, 李文刚, 王烁, 于婧. 基于专利数据分析热熔挤出技术在生物医药领域的应用[J]. 生物技术进展, 2024, 14(2): 331-337. |
| [2] | 孙瑞雪, 米薇, 叶子弘. 基于质谱技术的蛋白质互作研究进展[J]. 生物技术进展, 2022, 12(2): 161-167. |
| [3] | 张晓龙,李立平,鲁仁义,阎澜,姜远英. 基于iTRAQ技术分析五倍子作用白念珠菌后的差异蛋白表达[J]. 生物技术进展, 2018, 8(6): 537-545. |
| [4] | 秦海波,朱建明. 中国典型高硒区硒的环境地球化学研究进展[J]. 生物技术进展, 2017, 7(5): 367-373. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部