


生物技术进展 ›› 2025, Vol. 15 ›› Issue (4): 573-586.DOI: 10.19586/j.2095-2341.2025.0055
• 进展评述 • 上一篇
刘闯1,2( ), 马建爽2, 柏映国2, 黄火清2, 涂涛2, 于会民2, 刘宁1(
), 马建爽2, 柏映国2, 黄火清2, 涂涛2, 于会民2, 刘宁1( ), 王苑2(
), 王苑2( )
)
收稿日期:2025-04-29
接受日期:2025-06-03
出版日期:2025-07-25
发布日期:2025-09-08
通讯作者:
刘宁,王苑
作者简介:刘闯 E-mail: liuchuang20252025@163.com
基金资助:
Chuang LIU1,2( ), Jianshuang MA2, Yingguo BAI2, Huoqing HUANG2, Tao TU2, Huimin YU2, Ning LIU1(
), Jianshuang MA2, Yingguo BAI2, Huoqing HUANG2, Tao TU2, Huimin YU2, Ning LIU1( ), Yuan WANG2(
), Yuan WANG2( )
)
Received:2025-04-29
Accepted:2025-06-03
Online:2025-07-25
Published:2025-09-08
Contact:
Ning LIU,Yuan WANG
摘要:
豆粕作为重要的植物蛋白来源,在饲料和食品工业中应用广泛。然而,豆粕中抗营养因子(如抗原蛋白、非淀粉多糖等)的存在限制了其营养价值。酶解加工工艺是近年来新兴的一种改善豆粕营养价值的原料体外预处理方法,常用的酶制剂种类包括蛋白酶、纤维素酶、半乳糖苷酶等。总结了豆粕中抗营养因子的种类、不同的酶制剂及其作用机制、酶制剂的应用研究进展,旨在为豆粕酶解工艺中酶制剂的选择提供理论依据和技术参考。
中图分类号:
刘闯, 马建爽, 柏映国, 黄火清, 涂涛, 于会民, 刘宁, 王苑. 豆粕酶解工艺中常用酶制剂的酶学性质研究进展[J]. 生物技术进展, 2025, 15(4): 573-586.
Chuang LIU, Jianshuang MA, Yingguo BAI, Huoqing HUANG, Tao TU, Huimin YU, Ning LIU, Yuan WANG. Research Progress on the Enzymatic Properties of Common Enzyme Preparations in Soybean Meal Enzyme Hydrolysis Processes[J]. Current Biotechnology, 2025, 15(4): 573-586.
| 样品名称 | 大豆球蛋白/(mg·g-1) | β-伴大豆球蛋白/(mg·g-1) | 水苏糖/(mg·g-1 ) |
|---|---|---|---|
| 酶解豆粕 | 0.13~62.78 | 1.74~53.54 | 0.40~34.52 |
| 普通豆粕 | 58.90~204.30 | 42.80~185.80 | 16.59~48.67 |
表1 酶解豆粕与普通豆粕的大豆球蛋白、β-伴大豆球蛋白和水苏糖含量对比[2]
Table 1 Comparative analysis of glycinin, β-conglycinin, and stachyose contents in enzymatically hydrolyzed soybean meal versus conventional soybean meal[2]
| 样品名称 | 大豆球蛋白/(mg·g-1) | β-伴大豆球蛋白/(mg·g-1) | 水苏糖/(mg·g-1 ) |
|---|---|---|---|
| 酶解豆粕 | 0.13~62.78 | 1.74~53.54 | 0.40~34.52 |
| 普通豆粕 | 58.90~204.30 | 42.80~185.80 | 16.59~48.67 |
| 名称 | 来源 | 种类 | 底物偏好性 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 胰凝乳蛋白酶 | 金黄色葡萄球菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 8.5/5.0~10.0 | 100 h | 40/40~60 | 5 h | [ |
| 胰凝乳蛋白酶 | 日本鳗鲡 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 8.0/4.5~11.0 | 30 min | 40/30 | 60 min | [ |
| 胰蛋白酶 | 动物(胰腺) | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.5-8.5/7.0-9.0 | ND | 37~40/30~45 | ND | [ |
| 弹性蛋白酶 | 黑曲霉 | 丝氨酸蛋白酶 | 作用于小侧链疏水氨基酸(如丙氨酸、缬氨酸、亮氨酸)的羧基端肽键,尤其适合切割弹性蛋白等富含此类氨基酸的底物 | ND | ND | ND | ND | [ |
| 弹性蛋白酶 | 黄曲霉 | 丝氨酸蛋白酶 | ||||||
| 弹性蛋白酶 | 猪胰脏 | 丝氨酸蛋白酶 | ||||||
| 弹性蛋白酶 | 地衣芽孢杆菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.4/ND | ND | 55/ND | ND | [ |
| 弹性蛋白酶 | 短芽孢杆菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 9.5/7.0~11.5 | 1 h | 20/40~50 | 1 h | [ |
| 弹性蛋白酶 | 铜绿假单胞菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.4/3.0~5.0 | 4 h | 28~37/28~37 | 3 h | [ |
| 枯草杆菌蛋白酶 | 枯草杆菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 7.0/6.0~8.0 | 12 h | 60~70/20~40 | 1 h | [ |
| SPRK蛋白酶 K | 沙雷氏菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 10.5/ND | 24 h | 70/70 | 19 min | [ |
| AQUI蛋白酶 K | 水生嗜热杆菌YT-1 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | ND | ND | 80/70~80 | 30 min | [ |
| TPRK蛋白酶 K | 林伯氏白色念球菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 9.0/7.0~11.0 | 2 d | 65/45~55 | 100 min | [ |
| 嗜热蛋白酶 Tth0724 | 极端嗜热细菌HB8 | 丝氨酸蛋白酶 | 疏水性/中性蛋白底物具有较强水解能力 | 7.0/ND | ND | 70~80/80 | 150 min | [ |
| 胃蛋白酶 | ND | 天冬氨酸蛋白酶 | 疏水氨基酸(如苯丙氨酸、酪氨酸、亮氨酸、异亮氨酸)的氨基端肽键 | 2.0/2.0~5.0 | 1 h | 60/55~70 | 30 min | [ |
| 胃蛋白酶 | 动物(胃) | 天冬氨酸蛋白酶 | 疏水氨基酸(如苯丙氨酸、酪氨酸、亮氨酸、异亮氨酸)的氨基端肽键 | 2.0~3.0 | 1.5~4.0 | 37~40/ND | 30 min | [ |
| 嗜热菌蛋白酶 | 嗜热解蛋白芽孢杆菌 | 金属蛋白酶 | 疏水性氨基酸(亮氨酸、苯丙氨酸) | 7.0/ND | ND | 70~80/80 | 150 min | [ |
| 嗜热菌蛋白酶 | 无氧芽胞杆菌属 | 金属蛋白酶 | 疏水氨基酸(如亮氨酸、异亮氨酸、缬氨酸、苯丙氨酸)的羧基端肽键,尤其偏好脂肪族疏水残基 | 7.0/ND | ND | 55/ND | ND | [ |
| 组织蛋白酶L | 牛胰脏 | 半胱氨酸蛋白酶 | 疏水氨基酸(如亮氨酸、苯丙氨酸)相邻的肽键有较高亲和力 | 6.5/3.0~8.0 | 1 h | 40~50/40 | 1 h | [ |
| 组织蛋白酶L | 虾肝胰腺 | 半胱氨酸蛋白酶 | 疏水氨基酸(如亮氨酸、苯丙氨酸)相邻的肽键有较高亲和力 | 5.5/5.5~6.5 | 30 min | 35/4~30 | 30 min | [ |
| 组织蛋白酶B | 红鳍东方鲀 | 半胱氨酸蛋白酶 | ND | 7.0~8.0/3.5~8.0 | 30 min | 45~50/20~50 | 30 min | [ |
| 组织蛋白酶 L | 红鳍东方鲀 | 半胱氨酸蛋白酶 | ND | 6.0~7.0/3.5~6.5 | 30 min | 45~50/20~50 | 30 min | [ |
| 木瓜蛋白酶 | 木瓜乳汁 | 半胱氨酸蛋白酶 | 广谱水解,偏好疏水/芳香族氨基酸(苯丙氨酸、酪氨酸)及酰基转移反应 | 6.0~7.0/4.0~9.0 | ND | 40~60/≤60 | 30 min | [ |
| 木瓜蛋白酶 | 木瓜乳汁 | 半胱氨酸蛋白酶 | 广谱水解(偏好疏水/芳香族氨基酸) | 7.0/5.0~9.0 | ND | 50/≤60 | 90 min | [ |
表2 不同蛋白酶的分类及特性
Table 2 Classification and characteristics of different proteases
| 名称 | 来源 | 种类 | 底物偏好性 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 胰凝乳蛋白酶 | 金黄色葡萄球菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 8.5/5.0~10.0 | 100 h | 40/40~60 | 5 h | [ |
| 胰凝乳蛋白酶 | 日本鳗鲡 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 8.0/4.5~11.0 | 30 min | 40/30 | 60 min | [ |
| 胰蛋白酶 | 动物(胰腺) | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.5-8.5/7.0-9.0 | ND | 37~40/30~45 | ND | [ |
| 弹性蛋白酶 | 黑曲霉 | 丝氨酸蛋白酶 | 作用于小侧链疏水氨基酸(如丙氨酸、缬氨酸、亮氨酸)的羧基端肽键,尤其适合切割弹性蛋白等富含此类氨基酸的底物 | ND | ND | ND | ND | [ |
| 弹性蛋白酶 | 黄曲霉 | 丝氨酸蛋白酶 | ||||||
| 弹性蛋白酶 | 猪胰脏 | 丝氨酸蛋白酶 | ||||||
| 弹性蛋白酶 | 地衣芽孢杆菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.4/ND | ND | 55/ND | ND | [ |
| 弹性蛋白酶 | 短芽孢杆菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 9.5/7.0~11.5 | 1 h | 20/40~50 | 1 h | [ |
| 弹性蛋白酶 | 铜绿假单胞菌 | 丝氨酸蛋白酶 | 疏水性芳香族氨基酸(苯丙氨酸、酪氨酸、色氨酸) | 7.4/3.0~5.0 | 4 h | 28~37/28~37 | 3 h | [ |
| 枯草杆菌蛋白酶 | 枯草杆菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 7.0/6.0~8.0 | 12 h | 60~70/20~40 | 1 h | [ |
| SPRK蛋白酶 K | 沙雷氏菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 10.5/ND | 24 h | 70/70 | 19 min | [ |
| AQUI蛋白酶 K | 水生嗜热杆菌YT-1 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | ND | ND | 80/70~80 | 30 min | [ |
| TPRK蛋白酶 K | 林伯氏白色念球菌 | 丝氨酸蛋白酶 | 对疏水氨基酸(如亮氨酸、异亮氨酸)附近的肽键有较高活性 | 9.0/7.0~11.0 | 2 d | 65/45~55 | 100 min | [ |
| 嗜热蛋白酶 Tth0724 | 极端嗜热细菌HB8 | 丝氨酸蛋白酶 | 疏水性/中性蛋白底物具有较强水解能力 | 7.0/ND | ND | 70~80/80 | 150 min | [ |
| 胃蛋白酶 | ND | 天冬氨酸蛋白酶 | 疏水氨基酸(如苯丙氨酸、酪氨酸、亮氨酸、异亮氨酸)的氨基端肽键 | 2.0/2.0~5.0 | 1 h | 60/55~70 | 30 min | [ |
| 胃蛋白酶 | 动物(胃) | 天冬氨酸蛋白酶 | 疏水氨基酸(如苯丙氨酸、酪氨酸、亮氨酸、异亮氨酸)的氨基端肽键 | 2.0~3.0 | 1.5~4.0 | 37~40/ND | 30 min | [ |
| 嗜热菌蛋白酶 | 嗜热解蛋白芽孢杆菌 | 金属蛋白酶 | 疏水性氨基酸(亮氨酸、苯丙氨酸) | 7.0/ND | ND | 70~80/80 | 150 min | [ |
| 嗜热菌蛋白酶 | 无氧芽胞杆菌属 | 金属蛋白酶 | 疏水氨基酸(如亮氨酸、异亮氨酸、缬氨酸、苯丙氨酸)的羧基端肽键,尤其偏好脂肪族疏水残基 | 7.0/ND | ND | 55/ND | ND | [ |
| 组织蛋白酶L | 牛胰脏 | 半胱氨酸蛋白酶 | 疏水氨基酸(如亮氨酸、苯丙氨酸)相邻的肽键有较高亲和力 | 6.5/3.0~8.0 | 1 h | 40~50/40 | 1 h | [ |
| 组织蛋白酶L | 虾肝胰腺 | 半胱氨酸蛋白酶 | 疏水氨基酸(如亮氨酸、苯丙氨酸)相邻的肽键有较高亲和力 | 5.5/5.5~6.5 | 30 min | 35/4~30 | 30 min | [ |
| 组织蛋白酶B | 红鳍东方鲀 | 半胱氨酸蛋白酶 | ND | 7.0~8.0/3.5~8.0 | 30 min | 45~50/20~50 | 30 min | [ |
| 组织蛋白酶 L | 红鳍东方鲀 | 半胱氨酸蛋白酶 | ND | 6.0~7.0/3.5~6.5 | 30 min | 45~50/20~50 | 30 min | [ |
| 木瓜蛋白酶 | 木瓜乳汁 | 半胱氨酸蛋白酶 | 广谱水解,偏好疏水/芳香族氨基酸(苯丙氨酸、酪氨酸)及酰基转移反应 | 6.0~7.0/4.0~9.0 | ND | 40~60/≤60 | 30 min | [ |
| 木瓜蛋白酶 | 木瓜乳汁 | 半胱氨酸蛋白酶 | 广谱水解(偏好疏水/芳香族氨基酸) | 7.0/5.0~9.0 | ND | 50/≤60 | 90 min | [ |
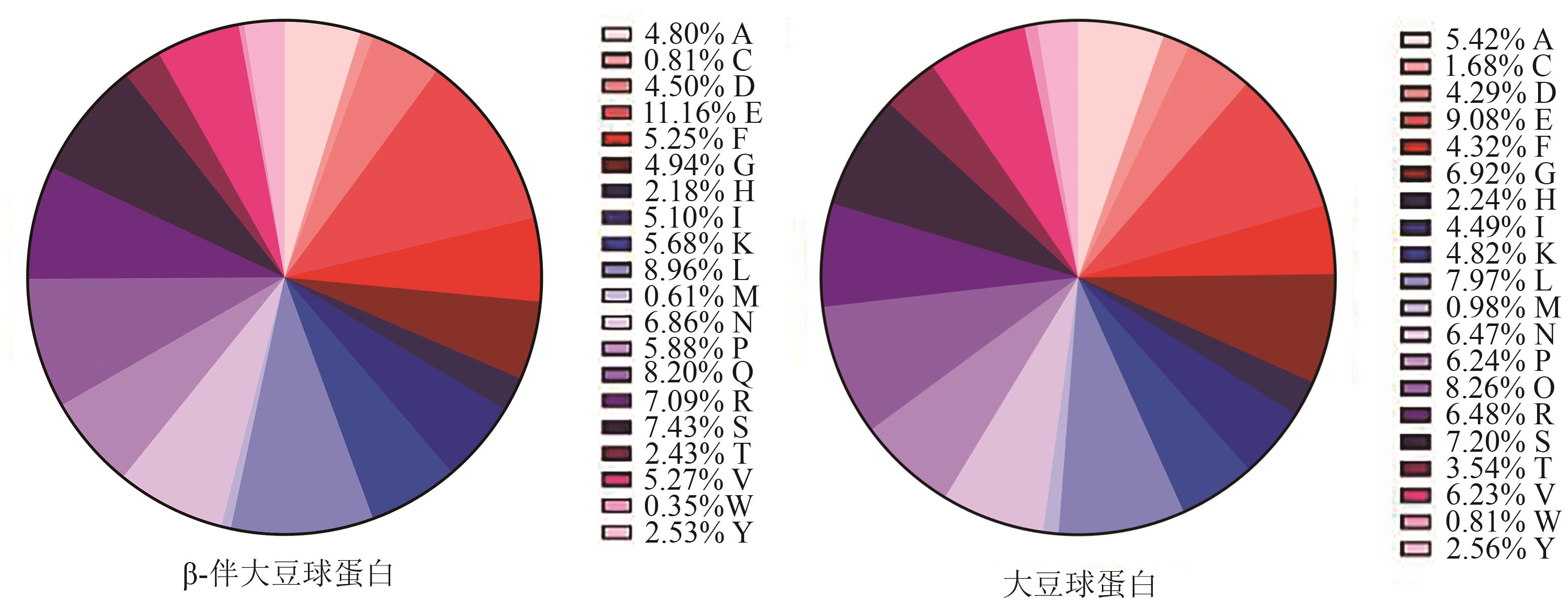
图1 β-伴大豆球蛋白、大豆球蛋白的氨基酸含量注:图中字母代表氨基酸;A—丙氨酸;C—半胱氨酸;D—天冬氨酸;E—谷氨酸;F—苯丙氨酸;G—甘氨酸;H—组氨酸;I—异亮氨酸;K—赖氨酸;L—亮氨酸;M—甲硫氨酸;N—天冬酰胺;P—脯氨酸;Q—谷氨酰胺;R—精氨酸;S—丝氨酸;T—苏氨酸;V—缬氨酸;W—色氨酸;Y—络氨酸。
Fig. 1 Amino acid composition of β-conglycinin and glycinin
| 类别 | 氨基酸组成 | β-伴大豆球蛋白含量/% | 氨基酸总和/% | 大豆球蛋白含量/% | 氨基酸总和/% |
|---|---|---|---|---|---|
| 疏水 | A(丙氨酸) | 4.80 | 41.16 | 5.42 | 43.38 |
| F(苯丙氨酸) | 5.25 | 4.32 | |||
| I(异亮氨酸) | 5.10 | 4.49 | |||
| L(亮氨酸) | 8.96 | 7.97 | |||
| M(甲硫氨酸) | 0.61 | 0.98 | |||
| P(脯氨酸) | 5.88 | 6.24 | |||
| V(缬氨酸) | 5.27 | 6.23 | |||
| G(甘氨酸) | 4.94 | 6.92 | |||
| W(色氨酸) | 0.35 | 0.81 | |||
| 带电荷 | D(天冬氨酸) | 4.50 | 30.61 | 4.29 | 26.91 |
| E(谷氨酸) | 11.20 | 9.08 | |||
| K(赖氨酸) | 5.68 | 4.82 | |||
| R(精氨酸) | 7.09 | 6.48 | |||
| H(组氨酸) | 2.18 | 2.24 | |||
| 带羟基 | S(丝氨酸) | 7.43 | 12.39 | 7.20 | 13.3 |
| T(苏氨酸) | 2.43 | 3.54 | |||
| Y(酪氨酸) | 2.53 | 2.56 | |||
| 带苯环 | F(苯丙氨酸) | 5.25 | 8.13 | 4.32 | 7.69 |
| W(色氨酸) | 0.35 | 0.81 | |||
| Y(酪氨酸) | 2.53 | 2.56 |
表3 对氨基酸的R基特性和带电特性进行分组统计
Table 3 Grouping statistics based on R-group properties and charge characteristics of amino acids
| 类别 | 氨基酸组成 | β-伴大豆球蛋白含量/% | 氨基酸总和/% | 大豆球蛋白含量/% | 氨基酸总和/% |
|---|---|---|---|---|---|
| 疏水 | A(丙氨酸) | 4.80 | 41.16 | 5.42 | 43.38 |
| F(苯丙氨酸) | 5.25 | 4.32 | |||
| I(异亮氨酸) | 5.10 | 4.49 | |||
| L(亮氨酸) | 8.96 | 7.97 | |||
| M(甲硫氨酸) | 0.61 | 0.98 | |||
| P(脯氨酸) | 5.88 | 6.24 | |||
| V(缬氨酸) | 5.27 | 6.23 | |||
| G(甘氨酸) | 4.94 | 6.92 | |||
| W(色氨酸) | 0.35 | 0.81 | |||
| 带电荷 | D(天冬氨酸) | 4.50 | 30.61 | 4.29 | 26.91 |
| E(谷氨酸) | 11.20 | 9.08 | |||
| K(赖氨酸) | 5.68 | 4.82 | |||
| R(精氨酸) | 7.09 | 6.48 | |||
| H(组氨酸) | 2.18 | 2.24 | |||
| 带羟基 | S(丝氨酸) | 7.43 | 12.39 | 7.20 | 13.3 |
| T(苏氨酸) | 2.43 | 3.54 | |||
| Y(酪氨酸) | 2.53 | 2.56 | |||
| 带苯环 | F(苯丙氨酸) | 5.25 | 8.13 | 4.32 | 7.69 |
| W(色氨酸) | 0.35 | 0.81 | |||
| Y(酪氨酸) | 2.53 | 2.56 |
| 来源 | 底物 | 最适pH/耐受pH | pH耐受 时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mg-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 植物乳杆菌MTCC 5422 | 棉子糖 | 5.8/4.8~6.2 | 12 h | 45/55 | 30 min | 12.0 | [ |
| 莱氏篮状菌JCM12802 | pNPGal | 4.0/3.0~11.0 | 1 h | 70/65 | 1 h | 389.8 | [ |
| 链球菌S1 | pNPG | 6.5/7.5~10.0 | ND | 50/40 | 1 h | 508.3 | [ |
| 弯曲乳杆菌R08 | 水苏糖 | 6.5~7.0/6.5~10.5 | ND | 37/30~50 | 3 h | 2.7 | [ |
| 费氏新萨托菌P1 | pNPGal | 4.5/2.0~12.0 | 1 h | 60~70/60 | 10 min | 449.5 | [ |
| 黑曲霉变种 RM48 | pNPGal | 4.5/3.5~6.0 | 1 h | 60/40 | ND | 5 618.0 | [ |
| 酵母菌 | pNPGal | 4.0/ND | ND | 70/70 | 15 min | 100.0 | [ |
| 费氏新萨托菌P1 | pNPGal | 4.5/3.0~10.0 | 15 min | 60~70/70 | 15 min | 423.0 | [ |
| 双孢霉MEY-1 | pNPGal | 3.5/2.2~8.0 | 60 min | 55/65 | 60 min | 612.9 | [ |
| 巨大芽孢杆菌 | pNPGal | 5.0/4.0~9.0 | 60 min | 50/55 | 30 min | 303.0 | [ |
| 灰绿青霉 | 半乳寡糖 | 5.0/4.0~6.0 | 3 h | 65/60 | 3 h | 296.0 | [ |
表4 不同来源α-半乳糖苷酶的功能特性
Table 4 Functional characteristics of α-galactosidases from different sources
| 来源 | 底物 | 最适pH/耐受pH | pH耐受 时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mg-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 植物乳杆菌MTCC 5422 | 棉子糖 | 5.8/4.8~6.2 | 12 h | 45/55 | 30 min | 12.0 | [ |
| 莱氏篮状菌JCM12802 | pNPGal | 4.0/3.0~11.0 | 1 h | 70/65 | 1 h | 389.8 | [ |
| 链球菌S1 | pNPG | 6.5/7.5~10.0 | ND | 50/40 | 1 h | 508.3 | [ |
| 弯曲乳杆菌R08 | 水苏糖 | 6.5~7.0/6.5~10.5 | ND | 37/30~50 | 3 h | 2.7 | [ |
| 费氏新萨托菌P1 | pNPGal | 4.5/2.0~12.0 | 1 h | 60~70/60 | 10 min | 449.5 | [ |
| 黑曲霉变种 RM48 | pNPGal | 4.5/3.5~6.0 | 1 h | 60/40 | ND | 5 618.0 | [ |
| 酵母菌 | pNPGal | 4.0/ND | ND | 70/70 | 15 min | 100.0 | [ |
| 费氏新萨托菌P1 | pNPGal | 4.5/3.0~10.0 | 15 min | 60~70/70 | 15 min | 423.0 | [ |
| 双孢霉MEY-1 | pNPGal | 3.5/2.2~8.0 | 60 min | 55/65 | 60 min | 612.9 | [ |
| 巨大芽孢杆菌 | pNPGal | 5.0/4.0~9.0 | 60 min | 50/55 | 30 min | 303.0 | [ |
| 灰绿青霉 | 半乳寡糖 | 5.0/4.0~6.0 | 3 h | 65/60 | 3 h | 296.0 | [ |
| 来源 | 底物 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 草酸青霉 | CMC-Na | 7.0/5.0~9.0 | 72 h | 28/24~32 | 72 h | 74.1 | [ |
| 点斑嗜菌霉 | CMC-Na | 5.5/4.0~9.0 | 1 h(52% 以上) | 55/80 | 15 min | 105.6 | [ |
| 贝莱斯芽孢杆菌 | CMC-Na | 5.5~6.5/4.0~9.5 | ND | 50/30~60 | ND | 125.9 | [ |
| 地衣芽孢杆菌 | CMC-Na | 7.0/5.5~7.5 | ND | 37/28~45 | ND | 181.2 | [ |
| 解脂耶氏酵母菌 | CMC-Na | 6.0/3.0~7.0 | 72 h | 30/30~39 | 72 h | 1.3 | [ |
| 棘孢木霉 | 蛋白胨 | 4.0/3.5~5.0 | 24 h | 50/45~55 | 30 min | 2.8 | [ |
| 枯草芽孢杆菌 | 蛋白胨 | 6.1/5.5~6.6 | 96 h | 37/34~40 | 96 h | 222.0 | [ |
| 白耙齿菌和栓孔菌 | 乳糖 | 6.0/4.0~8.0 | 4 d | 28/24~32 | 4 d | 112.1 | [ |
| 枯草芽孢杆菌 | CMC-Na | 6.0/5.0~7.5 | 2 d | 37/30~40 | 2 d | 39.0 | [ |
| 解淀粉芽孢杆菌植物亚种 | CMC-Na | 7.0/3.0~11.0 | 60 h | 37/33~41 | 60 h | 21.0 | [ |
| 贝莱斯芽孢杆菌 | CMC-Na | 5.26/ND | 6 d | 34.73/ND | 6 d | 233.5 | [ |
表5 不同来源纤维素酶的功能特性
Table 5 Functional characteristics of cellulases from different sources
| 来源 | 底物 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 草酸青霉 | CMC-Na | 7.0/5.0~9.0 | 72 h | 28/24~32 | 72 h | 74.1 | [ |
| 点斑嗜菌霉 | CMC-Na | 5.5/4.0~9.0 | 1 h(52% 以上) | 55/80 | 15 min | 105.6 | [ |
| 贝莱斯芽孢杆菌 | CMC-Na | 5.5~6.5/4.0~9.5 | ND | 50/30~60 | ND | 125.9 | [ |
| 地衣芽孢杆菌 | CMC-Na | 7.0/5.5~7.5 | ND | 37/28~45 | ND | 181.2 | [ |
| 解脂耶氏酵母菌 | CMC-Na | 6.0/3.0~7.0 | 72 h | 30/30~39 | 72 h | 1.3 | [ |
| 棘孢木霉 | 蛋白胨 | 4.0/3.5~5.0 | 24 h | 50/45~55 | 30 min | 2.8 | [ |
| 枯草芽孢杆菌 | 蛋白胨 | 6.1/5.5~6.6 | 96 h | 37/34~40 | 96 h | 222.0 | [ |
| 白耙齿菌和栓孔菌 | 乳糖 | 6.0/4.0~8.0 | 4 d | 28/24~32 | 4 d | 112.1 | [ |
| 枯草芽孢杆菌 | CMC-Na | 6.0/5.0~7.5 | 2 d | 37/30~40 | 2 d | 39.0 | [ |
| 解淀粉芽孢杆菌植物亚种 | CMC-Na | 7.0/3.0~11.0 | 60 h | 37/33~41 | 60 h | 21.0 | [ |
| 贝莱斯芽孢杆菌 | CMC-Na | 5.26/ND | 6 d | 34.73/ND | 6 d | 233.5 | [ |
| 来源 | 底物 | 最适pH/耐受pH | pH耐受 时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 微小杆菌属 | 水杨苷 | 6-7/5.0~8.0 | 1 h | 50~55/<50 | 1 h | ND | [ |
| 枯草芽孢杆菌 | 水杨苷 | 6.5/4.0~7.5 | 24 h | 60/40-65 | 1 h | 4.9 | [ |
| 酿酒酵母 | p-NPG | 5.0/ND | 10 min | 50/ND | 10 min | 51.4 | [ |
| 多粘芽孢杆菌 | p-NPG | 7.0/ND | 10 min | 37/ND | 10 min | 24.7 | [ |
| 木薯块根 | p-NPG | 7.0/6.0~8.0 | 24 h | 40/ND | 48 h(73.01%) | 1.1 | [ |
| 米曲霉 | 水杨素 | 4.5/4.0~6.0 | 1 h | 55/30~50 | 1 h | 40.8 | [ |
| 大豆 | 大豆异黄酮糖苷 | 5.0/5.0~7.0 | 24 h | 45/<40 | ND | 2.2 | [ |
| 芽枝霉菌 | 七叶苷 | 5.0/4.0~9.0 | ND | ND/<60 | ND | 7.2 | [ |
| 毕赤酵母属 | p-NPG | 5.0/ND | 15 min | 30/ND | 72 h | 276.1 | [ |
| 黑曲霉 | 人参皂苷-Rg3 | 5.0/3.0~7.0 | 18 h | 40/30~50 | ND | ND | [ |
| 曲霉菌 | p-NPG | 5.0/3.0~7.0 | 24 h | 50/20~80 | 4 h | 1 066.0 | [ |
表6 不同来源β-葡萄糖苷酶的的功能特性
Table 6 Functional characteristics of β-glucosidases from different sources
| 来源 | 底物 | 最适pH/耐受pH | pH耐受 时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 微小杆菌属 | 水杨苷 | 6-7/5.0~8.0 | 1 h | 50~55/<50 | 1 h | ND | [ |
| 枯草芽孢杆菌 | 水杨苷 | 6.5/4.0~7.5 | 24 h | 60/40-65 | 1 h | 4.9 | [ |
| 酿酒酵母 | p-NPG | 5.0/ND | 10 min | 50/ND | 10 min | 51.4 | [ |
| 多粘芽孢杆菌 | p-NPG | 7.0/ND | 10 min | 37/ND | 10 min | 24.7 | [ |
| 木薯块根 | p-NPG | 7.0/6.0~8.0 | 24 h | 40/ND | 48 h(73.01%) | 1.1 | [ |
| 米曲霉 | 水杨素 | 4.5/4.0~6.0 | 1 h | 55/30~50 | 1 h | 40.8 | [ |
| 大豆 | 大豆异黄酮糖苷 | 5.0/5.0~7.0 | 24 h | 45/<40 | ND | 2.2 | [ |
| 芽枝霉菌 | 七叶苷 | 5.0/4.0~9.0 | ND | ND/<60 | ND | 7.2 | [ |
| 毕赤酵母属 | p-NPG | 5.0/ND | 15 min | 30/ND | 72 h | 276.1 | [ |
| 黑曲霉 | 人参皂苷-Rg3 | 5.0/3.0~7.0 | 18 h | 40/30~50 | ND | ND | [ |
| 曲霉菌 | p-NPG | 5.0/3.0~7.0 | 24 h | 50/20~80 | 4 h | 1 066.0 | [ |
| 来源 | 底物 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 重组嗜酸乳杆菌 | 甘露聚糖 | 5.5/5.0~7.0 | ND | 55/50~60 | ND | 353.0 | [ |
| 枯草芽孢杆菌 | 刺槐豆胶 | 6.0/5.5~7.0 | ND | 37/20~50 | ND | 15 265.0 | [ |
| 重组豆曲霉 AsT1 | 咖啡提取物 | 5.0/3.0~7.0 | ND | 40/40~60 | ND | 180.0 | [ |
| 重组米曲霉 MTCC 1846 | 角豆粉 | 5.0/3.0~7.0 | 4 h | 60/30~90 | ND | 43.4 | [ |
| 宇佐美曲霉 | 魔芋粉 | 5.0~8.0 | ND | 31/25~34 | ND | 3 362.0 | [ |
| 地衣芽孢杆菌 | 角豆胶 | 5.0/4.0~6.0 | ND | 60/50~65 | ND | ND | [ |
| 枯草芽孢杆菌 SA-22 | 魔芋粉 | 6.5/5.0~10.0 | ND | 70/50~70 | 4 h | ND | [ |
| 芽孢杆菌 M50 | 魔芋粉 | 6.0/5.0~7.0 | 4 h | 50/25~65 | 15 min | 330.0 | [ |
| 枯草芽孢杆菌 CY S4 | 魔芋胶 | 7.0/5.0~11.0 | 2 h | 60/20~60 | 3 h | 11.0 | [ |
| 类芽孢杆菌 LLZ1 | 角豆胶 | 7.0 | ND | 50/ND | ND | 39.1 | [ |
| 地衣芽孢杆菌HDYM-04 | 魔芋粉 | 8.0 | ND | 37/ND | ND | 4 890.0 | [ |
| 酸乳酸片球菌 M17 | 刺槐豆胶 | 6.0/2.0~9.0 | ND | 60/30~80 | ND | 78.7 | [ |
表7 不同来源甘露聚糖酶的的功能特性
Table 7 Functional characteristics of mannanases from different sources
| 来源 | 底物 | 最适pH/耐受pH | pH耐受时间 | 最适温度/耐受温度/℃ | 高温耐受时间 | 酶活/(U·mL-1) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 重组嗜酸乳杆菌 | 甘露聚糖 | 5.5/5.0~7.0 | ND | 55/50~60 | ND | 353.0 | [ |
| 枯草芽孢杆菌 | 刺槐豆胶 | 6.0/5.5~7.0 | ND | 37/20~50 | ND | 15 265.0 | [ |
| 重组豆曲霉 AsT1 | 咖啡提取物 | 5.0/3.0~7.0 | ND | 40/40~60 | ND | 180.0 | [ |
| 重组米曲霉 MTCC 1846 | 角豆粉 | 5.0/3.0~7.0 | 4 h | 60/30~90 | ND | 43.4 | [ |
| 宇佐美曲霉 | 魔芋粉 | 5.0~8.0 | ND | 31/25~34 | ND | 3 362.0 | [ |
| 地衣芽孢杆菌 | 角豆胶 | 5.0/4.0~6.0 | ND | 60/50~65 | ND | ND | [ |
| 枯草芽孢杆菌 SA-22 | 魔芋粉 | 6.5/5.0~10.0 | ND | 70/50~70 | 4 h | ND | [ |
| 芽孢杆菌 M50 | 魔芋粉 | 6.0/5.0~7.0 | 4 h | 50/25~65 | 15 min | 330.0 | [ |
| 枯草芽孢杆菌 CY S4 | 魔芋胶 | 7.0/5.0~11.0 | 2 h | 60/20~60 | 3 h | 11.0 | [ |
| 类芽孢杆菌 LLZ1 | 角豆胶 | 7.0 | ND | 50/ND | ND | 39.1 | [ |
| 地衣芽孢杆菌HDYM-04 | 魔芋粉 | 8.0 | ND | 37/ND | ND | 4 890.0 | [ |
| 酸乳酸片球菌 M17 | 刺槐豆胶 | 6.0/2.0~9.0 | ND | 60/30~80 | ND | 78.7 | [ |
| [1] | ADACHI M, KANAMORI J, MASUDA T, et al.. Crystal structure of soybean 11S globulin: glycinin A3B4 homohexamer[J]. Proc. Natl. Acad. Sci. USA., 2003, 100(12): 7395-7400. |
| [2] | 杨玉娟,姚怡莎,秦玉昌,等.豆粕与发酵豆粕中主要抗营养因子调查分析[J].中国农业科学,2016,49(3):573-580. |
| YANG Y J, YAO Y S, QIN Y C, et al.. Investigation and analysis of main AFN in soybean meal and fermented soybean meal[J]. Sci. Agric. Sin., 2016, 49(3): 573-580. | |
| [3] | MARUYAMA N, ADACHI M, TAKAHASHI K, et al.. Crystal structures of recombinant and native soybean β-conglycinin β homotrimers[J]. Eur. J. Biochem., 2001, 268(12): 3595-3604. |
| [4] | 王蕾,王雅馨,石亚伟.植物多肽类胰蛋白酶抑制剂降糖功效研究进展[J].食品工业科技,2021,42(22):406-412. |
| WANG L, WANG Y X, SHI Y W. Recent progress in hypoglycemic effect of plant protein trypsin inhibitors[J]. Sci. Technol. Food Ind., 2021, 42(22): 406-412. | |
| [5] | LIENER I E. Implications of antinutritional components in soybean foods[J]. Crit. Rev. Food Sci. Nutr., 1994, 34(1): 31-67. |
| [6] | SIRTORI C R, LOVATI M R. Soy proteins and cardiovascular disease[J]. Curr. Atheroscler. Rep., 2001, 3(1): 47-53. |
| [7] | MURDOCK L L, SHADE R E. Lectins and protease inhibitors as plant defenses against insects[J]. J. Agric. Food Chem., 2002, 50(22): 6605-6611. |
| [8] | OKEDIGBA A O, ROSSO M L, YU D, et al.. Comparative binding affinity analysis of soybean meal bowman-birk and kunitz trypsin inhibitors in interactions with animal serine proteases[J]. ACS Food Sci. Technol., 2023, 3(8): 1344-1352. |
| [9] | 张良慧.发酵豆粕在雏鸡日粮中应用的研究[D].新乡:河南科技学院,2022. |
| [10] | PAN L, ZHAO Y, YUAN Z, et al.. The integrins involved in soybean agglutinin-induced cell cycle alterations in IPEC-J2[J]. Mol. Cells, 2017, 40(2): 109-116. |
| [11] | ZHAO Y, QIN G, SUN Z, et al.. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets[J]. Int. J. Mol. Sci., 2011, 12(12): 8502-8512. |
| [12] | LI F, XIE Y, GAO X, et al.. Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production[J]. Electron. J. Biotechnol., 2020, 48: 29-35. |
| [13] | DASKIRAN M, TEETER R G, FODGE D, et al.. An evaluation of endo-β-D-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in β-mannan content 1[J]. Poult. Sci., 2004, 83(4): 662-668. |
| [14] | RITZ C W, HULET R M, SELF B B, et al.. Growth and intestinal morphology of male turkeys as influenced by dietary supplementation of amylase and xylanase[J]. Poult. Sci., 1995, 74(8): 1329-1334. |
| [15] | SALEEM R, SHAH S Z H, FATIMA M, et al.. Use of dietary β-mannanase supplementation to increase nutrient digestibility and improve growth of juvenile rohu (Labeo rohita) given a feed based on plant ingredients[J]. Aquac. Int., 2023, 31(3): 1789-1804. |
| [16] | 齐德生.饲用酶制剂发展概述[J].饲料工业,2011,32(12):29-31. |
| QI D S. Overview of the development of feed enzyme preparation[J]. Feed. Ind., 2011, 32(12): 29-31. | |
| [17] | BARRETT A J, TOLLE D P, RAWLINGS N D. Managing peptidases in the genomic era[J]. Biol. Chem., 2003, 384(6): 873-882. |
| [18] | 潘丽洁,王斌,潘力.胰凝乳蛋白酶SplB在枯草芽孢杆菌中的重组表达及应用[J].食品科学,2023,44(2):181-188. |
| PAN L J, WANG B, PAN L. Recombinant expression and application of chymotrypsin SplB in Bacillus subtilis [J]. Food Sci., 2023, 44(2): 181-188. | |
| [19] | 李辉.日本鳗鲡胰蛋白酶、胰凝乳蛋白酶的分离纯化及性质分析[D].厦门:集美大学,2015. |
| [20] | 钱方,邓岩,王凤翼,等.碱性内切蛋白酶水解大豆蛋白的研究[J].大连轻工业学院学报,2000,19(1):40-44. |
| QIAN F, DENG Y, WANG F Y, et al.. Hydrolysis of soybean protein by basin endo protease Alcalase[J]. J. Dalian Inst. Light Ind., 2000, 19(1): 40-44. | |
| [21] | 吴建中,赵谋明,宁正祥,等.酶法水解生产大豆多肽研究[J].粮油加工与食品机械,2003(1):45-47. |
| WU J Z, ZHAO M M, NING Z X, et al.. Study on soybean peptides produced by enzymatic hydrolysis[J]. Mach. Cereals Oil Food Process., 2003(1): 45-47. | |
| [22] | 宋涵玉.弹性蛋白酶在黑曲霉中的表达及促溶蛋白标签的筛选[D].哈尔滨:东北农业大学,2022. |
| [23] | 傅明亮,刘晓杰,刘婧,等.地衣芽孢杆菌弹性蛋白酶纯化和性质研究[J].食品科学,2011,32(7):216-219. |
| FU M L, LIU X J, LIU J, et al.. Purification and characteristics of elastase produced by Bacillus licheniformis [J]. Food Sci., 2011, 32(7): 216-219. | |
| [24] | 樊陈,王茂广,高兆建,等.产低温弹性蛋白酶菌株的高效诱变及酶学特性[J].食品科学,2013,34(19):190-194. |
| FAN C, WANG M G, GAO Z J, et al.. Highly efficient mutation of Bacillus brevis to obtain cold-active elastase-producing strain and enzymatic characterization[J]. Food Sci., 2013, 34(19): 190-194. | |
| [25] | 谷新晰,许文涛,黄昆仑,等.重组弹性蛋白酶诱导表达、纯化及酶学特性研究[J].食品科学,2009,30(19):227-231. |
| GU X X, XU W T, HUANG K L, et al.. Expression, purification and enzymological properties of recombinant elastase[J]. Food Sci., 2009, 30(19): 227-231. | |
| [26] | 牛树壹.枯草杆菌蛋白酶的分离纯化、酶学性质及体外溶栓性质[D].青岛:中国海洋大学,2014. |
| [27] | LARSEN A N, MOE E, HELLAND R, et al.. Characterization of a recombinantly expressed proteinase K-like enzyme from a psychrotrophic Serratia sp.[J]. FEBS J., 2006, 273(1): 47-60. |
| [28] | SAKAGUCHI M, TAKEZAWA M, NAKAZAWA R, et al.. Role of disulphide bonds in a thermophilic serine protease aqualysin I from Thermus aquaticus YT-1[J]. J. Biochem., 2008, 143(5): 625-632. |
| [29] | 杨子璇.蛋白酶K在毕赤酵母中的异源表达及应用初探[D].天津:天津科技大学,2023. |
| [30] | 轩小然.嗜热蛋白酶Tth0724的枯草杆菌表达及其应用[D].长春:吉林大学,2022. |
| [31] | 赵玥.胃蛋白酶活力和热稳定性理性设计研究[D].上海:华东师范大学,2021. |
| [32] | 王芬,陈飞飞,高为芳,等.小分子封闭提高固定化嗜热菌蛋白酶的热稳定性[J].生物加工过程,2013,11(6):47-52. |
| WANG F, CHEN F F, GAO W F, et al.. Enhancement of thermal stability of immobilized thermolysin using end-group blocking[J]. Chin. J. Bioprocess Eng., 2013, 11(6): 47-52. | |
| [33] | 应长青,洪心,李旭颖,等.产蛋白酶嗜热菌Anoxybacillus sp.紫外诱变研究[J].哈尔滨医科大学学报,2015,49(5):385-388. |
| YING C Q, HONG X, LI X Y, et al.. Research on UV mutagenesis of protease-producing strain of thermophilus Anoxybacillus sp.[J]. J. Harbin Med. Univ., 2015, 49(5): 385-388. | |
| [34] | 崔昱清,王复龙,崔保威,等.牛胰脏组织蛋白酶L的纯化和酶学性质[J].食品科学,2015,36(15):142-146. |
| CUI Y Q, WANG F L, CUI B W, et al.. Purification and characterization of cathepsin L from bovine pancreas[J]. Food Sci., 2015, 36(15): 142-146. | |
| [35] | 颜龙杰,沈建东,张凌晶,等.凡纳滨对虾组织蛋白酶L性质分析及其对肌肉蛋白的降解[J].食品科学,2017,38(22):34-40. |
| YAN L J, SHEN J D, ZHANG L J, et al.. Characterization of cathepsin L from litopenaeus vannamei and its effect on muscular protein degradation[J]. Food Sci., 2017, 38(22): 34-40. | |
| [36] | 李妍钰.红鳍东方鲀组织蛋白酶B、L的异源表达及粗酶液性质研究[D].上海:上海海洋大学,2017. |
| [37] | YSUNG H, CHEN H J, LIU T Y, et al.. Improvement of the functionality of soy protein by introduction of new thiol groups through a papain-catalyzed acylation[J]. J. Food Sci., 1983, 48(3): 708-711. |
| [38] | 梁杰,林国荣,周凤超,等.纳米磁球固定化木瓜蛋白酶的制备及酶学性质研究[J].食品与发酵工业,2024,50(8):161-168. |
| LIANG J, LIN G R, ZHOU F C, et al.. Preparation and enzymatic properties of papain immobilized by nano-magnetic spheres[J]. Food Ferment. Ind., 2024, 50(8): 161-168. | |
| [39] | 张佳佳.饲用α-半乳糖苷酶固体扩大培养及其应用研究[D].杭州:浙江大学,2010. |
| [40] | ANISHA G S. Biopharmaceutical applications of α-galactosidases[J]. Biotechnol. Appl. Biochem., 2023, 70(1): 257-267. |
| [41] | VETRISELVI P M, NARASIMHAN M K, SAMUEL M, et al.. Biodegradation of sugarcane bagasse biomass using recombinant alpha-galactosidase overexpressing whole-cell E.coli: a sustainable method of agricultural waste utilization[J/OL]. 3 Biotech, 2024, 14(10): 246[2025-07-18]. . |
| [42] | 魏淑英,陈艳.α-半乳糖苷酶在豆粕型日粮中的应用[J].饲料博览,2008(5):8-11. |
| WEI S Y, CHEN Y. Application of α-galactosidase in soybean meal diet[J]. Feed. Rev., 2008(5): 8-11. | |
| [43] | ROOPASHRI A N, VARADARAJ M C. Hydrolysis of flatulence causing oligosaccharides by α-d-galactosidase of a probiotic Lactobacillus plantarum MTCC5422 in selected legume flours and elaboration of probiotic attributes in soy-based fermented product[J]. Eur. Food Res. Technol., 2014, 239(1): 99-115. |
| [44] | WANG C, WANG H, MA R, et al.. Biochemical characterization of a novel thermophilic α-galactosidase from Talaromyces leycettanus JCM12802 with significant transglycosylation activity[J]. J. Biosci. Bioeng., 2016, 121(1): 7-12. |
| [45] | 刁文涛,向凌云,宁萌,等.α-半乳糖苷酶基因在大肠杆菌中的表达及酶学性质研究[J].中国酿造,2019,38(8):60-66. |
| DIAO W T, XIANG L Y, NING M, et al.. Expression and enzymatic properties of α-galactosidase gene in Escherichia coli [J]. China Brew., 2019, 38(8): 60-66. | |
| [46] | YOON M Y, HWANG H J. Reduction of soybean oligosaccharides and properties of α-D-galactosidase from Lactobacillus curvatus R08 and Leuconostoc mesenteriodes JK55[J]. Food Microbiol., 2008, 25(6): 815-823. |
| [47] | WANG H, SHI P, LUO H, et al.. A thermophilic α-galactosidase from Neosartorya fischeri P1 with high specific activity, broad substrate specificity and significant hydrolysis ability of soymilk[J]. Bioresour. Technol., 2014, 153: 361-364. |
| [48] | 许尧兴,李艳丽,柳永,等.黑曲霉变种RM48 α-半乳糖苷酶的分离纯化及其酶学性质研究[J].浙江大学学报(农业与生命科学版),2009,35(2):147-152. |
| XU Y X, LI Y L, LIU Y, et al.. Purification and characterization of α-galactosidase from Aspergillus niger v. Tiegh RM48[J]. J. Zhejiang Univ. Agric. Life Sci., 2009, 35(2): 147-152. | |
| [49] | CAVAZZONI V, ADAMI A, CRAVERI R. α-galactosidase from the yeast Candida javanica [J]. Appl. Microbiol. Biotechnol., 1987, 26(6): 555-559. |
| [50] | WANG H, LUO H, LI J, et al.. An α-galactosidase from an acidophilic Bispora sp. MEY-1 strain acts synergistically with β-mannanase[J]. Bioresour. Technol., 2010, 101(21): 8376-8382. |
| [51] | 贲培培.α-半乳糖苷酶高产菌株的筛选及其基因的克隆、表达、纯化和性质研究[D].南京:南京农业大学,2011. |
| [52] | SINITSYNA O A, FEDOROVA E A, VAKAR I M, et al.. Isolation and characterization of extracellular α-galactosidases from Penicillium canescens [J]. Biochem. Mosc., 2008, 73(1): 97-106. |
| [53] | HKO C, HTSAI C, LIN P H, et al.. Characterization and pulp refining activity of a Paenibacillus campinasensis cellulase expressed in Escherichia coli [J]. Bioresour. Technol., 2010, 101(20): 7882-7888. |
| [54] | 张清翠,石雅丽,刘安礼,等.外切纤维素酶的研究与应用进展[J].生物技术进展,2020,10(5):495-502. |
| ZHANG Q C, SHI Y L, LIU A L, et al.. Research and application progress of exocellulase[J]. Curr. Biotechnol., 2020, 10(5): 495-502. | |
| [55] | 柴秀娟.高产纤维素酶菌株的筛选、酶学性质及发酵工艺的研究[D].新疆石河子:石河子大学,2015. |
| [56] | 黄忠永.纤维素酶的研究现状及其在畜牧生产中的应用[J].当代畜禽养殖业,2018(10):10. |
| HUANG Z Y. Research status of cellulase and its application in animal husbandry production[J]. Mod. Anim. Husb., 2018(10): 10. | |
| [57] | 刘建民,连梦思,彭洁.一株产纤维素酶和木聚糖酶真菌的鉴定及产酶条件优化[J].基因组学与应用生物学,2025,44(2):182-191. |
| LIU J M, LIAN M S, PENG J. Identification of a fungus producing cellulase and xylanase and optimization of enzyme production conditions[J]. Genom. Appl. Biol., 2025, 44(2): 182-191. | |
| [58] | 王瑾,白静,赵晶,等.新型GH45家族纤维素酶的智能挖掘及其在里氏木霉中的高效表达[J].生物技术进展,2025,15(2):287-295. |
| WANG J, BAI J, ZHAO J, et al.. Intelligent mining of novel GH45 cellulases and its efficient expression in Trichoderma reesei [J]. Curr. Biotechnol., 2025, 15(2): 287-295. | |
| [59] | 吴诗丽,谢莹莹,段雪凝,等.产纤维素酶菌株的筛选及其酶学性质研究[J/OL].食品工业科技:1-14[2025-04-23].. |
| WU S L, XIE Y Y, DUAN X N, et al. Screening of cellulase-producing strains and study on their enzymatic properties[J/OL]. Sci. Technol. Food Ind.: 1-14[2025-04-23]. . | |
| [60] | 张智,王蒙爱,冯丽荣,等.纤维素酶产生菌的筛选及功能菌NF-101的全基因分析与产酶工艺优化[J].现代食品科技,2024,40(11):107-118. |
| ZHANG Z, WANG M A, FENG L R, et al.. Screening of cellulase-producing bacteria, whole-gene analysis, and optimization of the enzyme production process for the functional bacterium NF-101[J]. Mod. Food Sci. Technol., 2024, 40(11): 107-118. | |
| [61] | 冉光耀,唐佳代,赵益梅,等.一株产纤维素酶酵母菌的筛选、鉴定及产酶条件优化[J].中国酿造,2024,43(9):72-78. |
| RAN G Y, TANG J D, ZHAO Y M, et al.. Screening, identification and enzyme production conditions optimization of a cellulase-producing yeast[J]. China Brew., 2024, 43(9): 72-78. | |
| [62] | 王彩衣,夏靖雯,庞冰瑜,等.高产纤维素酶菌株的筛选、固态发酵条件优化及其酶学性质研究[J].中国酿造,2024,43(9):105-111. |
| WANG C Y, XIA J W, PANG B Y, et al.. Screening and optimization of solid-state fermentation conditions of high cellulase-producing strain and its enzymatic property[J]. China Brew., 2024, 43(9): 105-111. | |
| [63] | 孔蒙蒙,金静静,卢鹏,等.高产纤维素酶工程菌株产酶条件优化[J].生物技术进展,2024,14(6):1032-1041. |
| KONG M M, JIN J J, LU P, et al.. Optimization of enzyme production conditions of high-yielding cellulase engineering strains[J]. Curr. Biotechnol., 2024, 14(6): 1032-1041. | |
| [64] | 范嘉慧,王志,任俊达,等.2株真菌混合发酵高产纤维素酶条件优化[J].中国酿造,2024,43(6):239-244. |
| FAN J H, WANG Z, REN J D, et al.. Optimization of conditions for high-yield cellulase by 2 fungal strains mixed fermentation[J]. China Brew., 2024, 43(6): 239-244. | |
| [65] | 单冬冬,赵柔,黄婷,等.一株纤维素酶产生菌的筛选与产酶条件优化[J].现代农业科技,2024(17):125-128. |
| SHAN D D, ZHAO R, HUANG T, et al.. Screening of a cellulase-producing strain and optimization of enzymatic production conditions[J]. Mod. Agric. Sci. Technol., 2024(17): 125-128. | |
| [66] | 国立东,王永春,于纯淼,等.一株高产纤维素酶的解淀粉芽孢杆菌分离及产酶条件优化[J].中国食品添加剂,2020,31(7):53-60. |
| GUO L D, WANG Y C, YU C M, et al.. Isolation of a high yield cellulase Bacillus amyloliquefaciens strain and its enzyme production optimization[J]. China Food Addit., 2020, 31(7): 53-60. | |
| [67] | 韦燕琪,宁思敏,韦昌浩,等.高产纤维素酶产生菌的筛选、鉴定及发酵条件优化[J].中国饲料,2024(17):46-54. |
| WEI Y Q, NING S M, WEI C H, et al.. Screening and identification of cellulase-producing strain and optimization of the fermentation conditions[J]. China Feed., 2024(17): 46-54. | |
| [68] | 王昊宇.β-葡萄糖苷酶的研究及应用[J].中国高新区,2018(14):215. |
| WANG H Y. Research and application of β-glucosidase[J]. Sci. Technol. Ind. Parks, 2018(14): 215. | |
| [69] | 姜婷婷,王奕丁,王全,等.产β-葡萄糖苷酶芽孢杆菌降解豆粕的发酵条件优化及抗营养因子测定[J].饲料工业,2017,38(14):52-55. |
| JIANG T T, WANG Y D, WANG Q, et al.. Optimization of fermentation conditions and anti-nutritional factors for soybean meal degradation by Bacillus β-glucosidase[J]. Feed. Ind., 2017, 38(14): 52-55. | |
| [70] | 黄琴,朱婷,蒋承建,等.产β-葡萄糖苷酶的菌种的筛选,鉴定及其酶学特性[J].基因组学与应用生物学,2011,30(5):590-595. |
| HUANG Q, ZHU T, JIANG C J, et al.. Isolation, identification and the enzymatic characteristics of the strain producing β-glucosidase[J]. Genom. Appl. Biol., 2011, 30(5): 590-595. | |
| [71] | 覃宝山,何海燕,李燕婷,等.β-葡萄糖苷酶产生菌的筛选鉴定及其粗酶液酶学性质研究[J].中国酿造,2024,43(8):128-132. |
| QIN B S, HE H Y, LI Y T, et al.. Screening and identification of β-glucosidase-producing strain and enzymatic properties of crude enzyme[J]. China Brew., 2024, 43(8): 128-132. | |
| [72] | 侯晓瑞,王婧,杨学山,等.甘肃河西走廊葡萄酒产区高产β-葡萄糖苷酶酵母菌株筛选[J].食品科学,2014,35(23):139-143. |
| HOU X R, WANG J, YANG X S, et al.. Screening of yeast strains producing β-glucosidase from Hexi Corridor wine-producing regions of Gansu Province[J]. Food Sci., 2014, 35(23): 139-143. | |
| [73] | 赵云,刘伟丰,毛爱军,等.多粘芽孢杆菌 (Bacillus polymyxa) β-葡萄糖苷酶基因在大肠杆菌中的表达、纯化及酶学性质分析[J].生物工程学报,2004,20(5):741-744. |
| ZHAO Y, LIU W F, MAO A J, et al.. Expression, purification and enzymatic characterization of Bacillus polymyxa β-glucosidase gene(bglA) in Escherichia coli [J]. Chin. J. Biotechnol., 2004, 20(5): 741-744. | |
| [74] | 常祥祥,田永莉,颜娟,等.木薯块根中β-葡萄糖苷酶的分离纯化及酶学性质[J].食品工业科技,2023,44(3):141-147. |
| CHANG X X, TIAN Y L, YAN J, et al.. Isolation, purification and characterization of β-glucosidase from cassava roots[J]. Sci. Technol. Food Ind., 2023, 44(3): 141-147. | |
| [75] | 陈静,郝伟伟,王春梅,等.产β-葡萄糖苷酶真菌的筛选鉴定、纯化及酶学性质分析[J].食品科学,2013,34(5):191-196. |
| CHEN J, HAO W W, WANG C M, et al.. Screening and identification of β-glucosidase-producing fungi, and purification and enzymatic analysis[J]. Food Sci., 2013, 34(5): 191-196. | |
| [76] | 孙艳梅,张永忠,王伊强,等.大豆β-葡萄糖苷酶水解大豆异黄酮糖苷的研究[J].中国粮油学报,2006,21(2):86-89. |
| SUN Y M, ZHANG Y Z, WANG Y Q, et al.. Hydrolysis of soybean isoflavone glucosides by β-glucosidases from soybean[J]. J. Chin. Cereals Oils Assoc., 2006, 21(2): 86-89. | |
| [77] | 覃拥灵,何海燕,刘园园,等.产β-葡萄糖苷酶野生真菌的筛选鉴定及酶学性质研究[J].中国酿造,2012,31(3):53-57. |
| QIN Y L, HE H Y, LIU Y Y, et al.. Screening and identification of a wild β-glucosidase producing fungal and its enzymatic property[J]. China Brew., 2012, 31(3): 53-57. | |
| [78] | 张敏,李佳益,倪永清,等.产β-葡萄糖苷酶非酿酒酵母的筛选及酶学特性研究[J].中国酿造,2016,35(5):97-101. |
| ZHANG M, LI J Y, NI Y Q, et al.. Screening of β-glucosidase-producing non-Saccharomyces yeasts with and its enzymatic characteristics[J]. China Brew., 2016, 35(5): 97-101. | |
| [79] | 张丹,刘耀平,鱼红闪,等.人参皂苷β-葡萄糖苷酶的分离纯化及其酶学特性[J].应用与环境生物学报,2003,9(3):259-262. |
| ZHANG D, LIU Y P, YU H S, et al.. Purification ofginsenoside-β- glucoside hydrolase and its characteristics[J]. Chin. J. Appl. Environ. Biol., 2003, 9(3): 259-262. | |
| [80] | RIOU C, SALMON J M, VALLIER M J, et al.. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae [J]. Appl. Environ. Microbiol., 1998, 64(10): 3607-3614. |
| [81] | WANG X Y, ZHI L D, ZHANG Z S, et al.. Application of two crucial hemicellulolytic enzymes in fish diet: xylanase and mannanase[J/OL]. Anim. Feed. Sci. Technol., 2025, 319: 116178[2025-07-18]. . |
| [82] | 顾斌涛,郭建军,曾静,等.嗜酸乳杆菌表达载体的构建及其甘露聚糖酶的表达[J].中国酿造,2024,43(1):136-140. |
| GU B T, GUO J J, ZENG J, et al.. Construction of Lactobacillus acidophilus expression vector and expression of mannanase[J]. China Brew., 2024, 43(1): 136-140. | |
| [83] | 李传峥,白龙,高畅,等. B.amyloliquefaciens T12高产β-甘露聚糖酶发酵优化及降解产物益生元活性研究[J].现代畜牧兽医,2024(5):23-30. |
| LI C Z, BAI L, GAO C, et al.. Fermentation optimization of high-yield β-mannanase from B. amyloliquefaciens T12 and prebiotic activity of degradation product[J]. Mod. J. Anim. Husb. Vet. Med., 2024(5): 23-30. | |
| [84] | ERKAN S B, BASMAK S, OZCAN A, et al.. Mannooligosaccharide production by β-mannanase enzyme application from coffee extract[J/OL]. J. Food Process. Preserv., 2021, 45(8): e14668[2025-07-17]. . |
| [85] | JANA U K, SURYAWANSHI R K, PRAJAPATI B P, et al.. Production optimization and characterization of mannooligosaccharide generating β-mannanase from Aspergillus oryzae [J]. Bioresour. Technol., 2018, 268: 308-314. |
| [86] | 李剑芳,邬敏辰,夏文水.β-甘露聚糖酶高产菌株选育及产酶条件的研究[J].食品与发酵工业,2005,31(9):9-13. |
| LI J F, WU M C, XIA W S. Breeding of acidic β-mannanase overproducing strain and studies on its enzyme production conditions[J]. Food Ferment. Ind., 2005, 31(9): 9-13. | |
| [87] | 刘朝辉,武伟娜,刘跃,等.保护剂提高β-甘露聚糖酶热稳定性的研究[J].天津大学学报,2008,41(1):114-118. |
| LIU Z H, WU W N, LIU Y, et al.. Enhancing the thermostability of β-mannanase by protective additives[J]. J. Tianjin Univ., 2008, 41(1): 114-118. | |
| [88] | YU H Y, SUN Y M, WANG W J, et al.. Purification and properties of Bacillus subtilis SA-22 endo-1, 4-β-D-mannanase[J]. Chin. J. Biotechnol., 2003, 19(3): 327-331. |
| [89] | 陈一平,龙健儿,廖连华,等.芽孢杆菌M50产生β甘露聚糖酶的条件研究[J].微生物学报,2000,40(1):62-68. |
| CHEN Y P, LONG J E, LIAO L H, et al.. Study on the production of βMANNANASE by Bacillus M50 [J]. Acta Microbiol. Sin., 2000, 40(1): 62-68. | |
| [90] | 李长影,孔雯,王家昕,等.β-甘露聚糖酶产生菌的分离鉴定和酶学性质[J].华中农业大学学报,2011,30(2):138-142. |
| LI C Y, KONG W, WANG J X, et al.. Isolation, identification and enzymatic characterization of β-mannanase producing strain[J]. J. Huazhong Agric. Univ., 2011, 30(2): 138-142. | |
| [91] | ZHOU T, JU X, YAN L, et al.. Production of mannooligosaccharides from orange peel waste with β-mannanase expressed in Trichosporonoides oedocephalis [J/OL]. Bioresour. Technol., 2024, 395: 130373[2025-07-18]. . |
| [92] | 葛菁萍,赵丹,宋刚,等.β-甘露聚糖酶产生菌HDYM-04的分离鉴定及产酶条件优化[J].食品科学,2009,30(21):262-266. |
| GE J P, ZHAO D, SONG G, et al.. Isolation and identification of beta-mannanase producing strain HDYM-04 and optimization of fermentation conditions[J]. Food Sci., 2009, 30(21): 262-266. | |
| [93] | NADAROGLU H, ADIGUZEL G, ADIGUZEL A, et al.. A thermostable-endo-β-(1, 4)-mannanase from Pediococcus acidilactici (M17): purification, characterization and its application in fruit juice clarification[J]. Eur. Food Res. Technol., 2017, 243(2): 193-201. |
| [94] | 赵龙妹,陈林,杜东晓,等.产纤维素酶细菌的筛选鉴定与特性分析[J].中国农学通报,2021,37(30):83-88. |
| ZHAO L M, CHEN L, DU D X, et al.. Screening, identification and characteristic analysis of cellulase-producing bacteria[J]. Chin. Agric. Sci. Bull., 2021, 37(30): 83-88. | |
| [95] | HUANG C H, HUANG T L, LIU Y C, et al.. Overexpression of a multifunctional β-glucosidase gene from thermophilic archaeon Sulfolobus solfataricus in transgenic tobacco could facilitate glucose release and its use as a reporter[J]. Transgenic. Res., 2020, 29(5-6): 511-527. |
| [96] | 李晨辉.产β-葡萄糖苷酶菌株的筛选鉴定及亚麻籽饼粕脱毒工艺的研究[D].兰州:兰州大学,2017. |
| [97] | ZHU M, ZHANG L, YANG F, et al.. A recombinant β-mannanase from Thermoanaerobacterium aotearoense SCUT27: biochemical characterization and its thermostability improvement[J]. J. Agric. Food Chem., 2020, 68(3): 818-825. |
| [98] | 胡学智,王俊.蛋白酶生产和应用的进展[J].工业微生物,2008,38(4):49-61. |
| HU X Z, WANG J. Advances in protease production and its applications[J]. Ind. Microbiol., 2008, 38(4): 49-61. | |
| [99] | ZHOU X, SUN R, ZHAO J, et al.. Enzymatic activity and stability of soybean oil body emulsions recovered under neutral and alkaline conditions: impacts of thermal treatments[J/OL]. LWT, 2022, 153: 112545[2025-07-18]. . |
| [100] | QIN J, ZOU X, LYU S, et al.. Influence of ionic liquids on lipase activity and stability in alcoholysis reactions[J]. RSC Adv., 2016, 6(90): 87703-87709. |
| [101] | MARTINOVIĆ T, ANDJELKOVIĆ U, GAJDOŠIK M Š, et al.. Foodborne pathogens and their toxins[J]. J. Proteomics, 2016, 147: 226-235. |
| [102] | 白燕.饲用蛋白酶体外评价及其在肉仔鸡日粮中的应用研究[D].呼和浩特:内蒙古农业大学,2015. |
| [103] | LIU H, WANG Y, ZHU D, et al.. Bioaccessibility and application of soybean isoflavones: a review[J]. Food Rev. Int., 2023, 39(8): 5948-5967. |
| [104] | HUANG J, HOU Q, YANG Y. Replacing hydrolyzed soybean meal with recombinant β-glucosidase enhances resistance to Clostridium perfringens in broilers through immune modulation[J/OL]. Int. J. Mol. Sci., 2024, 25(21): 11700[2025-07-18]. . |
| [105] | NINGRUM A, WARDANI D W, VANIDIA N, et al.. Evaluation of antioxidant activities from a sustainable source of okara protein hydrolysate using enzymatic reaction[J/OL]. Molecules, 2023, 28(13): 4974[2025-07-18]. . |
| [106] | HENG X, CHEN H, LU C, et al.. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases[J/OL]. LWT, 2022, 154: 112626[2025-07-17]. . |
| [107] | KETAREN P P, BATTERHAM E S, DETTMANN E B, et al.. Phosphorus studies in pigs[J]. Br. J. Nutr., 1993, 70(1): 289-311. |
| [1] | 刘晓敏, 卢婷, 李勇, 王猛, 朱保昆, 张伟. 酶制剂预处理对酵母菌发酵烟叶的影响[J]. 生物技术进展, 2025, 15(1): 93-101. |
| [2] | 王珍瑜, 陆文超, 钟康荣, 关永健, 汪真, 陈超. pH渐变条件下多酶分步连续酶解工艺制备鲟鱼硫酸软骨素与胶原蛋白肽[J]. 生物技术进展, 2023, 13(6): 934-939. |
| [3] | 胡佳瑶,张梅妍,王振灵,魏娜,高炳淼. 海马总蛋白提取及其酶解条件优化[J]. 生物技术进展, 2017, 7(4): 310-314. |
| [4] | 沈丹彤,李黎明,薛慧君,杨柳. 酶解辅助水蒸汽蒸馏法提取紫枝玫瑰精油工艺研究[J]. 生物技术进展, 2017, 7(2): 161-165. |
| [5] | 王林,朱金峰,许自成,张景华. 不同酶制剂对烤烟上部叶化学成分、游离态和糖苷结合态中性香气成分的影响[J]. 生物技术进展, 2015, 5(6): 455-460. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部