


生物技术进展 ›› 2024, Vol. 14 ›› Issue (4): 555-565.DOI: 10.19586/j.2095-2341.2024.0002
• 进展评述 • 上一篇
收稿日期:2024-01-09
接受日期:2024-05-30
出版日期:2024-07-25
发布日期:2024-08-07
作者简介:毕瑞 E-mail: 14901932@qq.com
基金资助:
Rui BI1( ), Jiangbo WU2, Chunjing MA1
), Jiangbo WU2, Chunjing MA1
Received:2024-01-09
Accepted:2024-05-30
Online:2024-07-25
Published:2024-08-07
摘要:
细胞表面成分与结构在维持细胞内代谢环境稳定、控制细胞内外物质交换和促进细胞间通讯方面发挥着至关重要的作用,因此,调节或改变细胞表面成分对研究细胞命运具有重要意义。基因工程是目前调节细胞表面成分最常用的技术,但是由于这种方法具有转染效率低、存在突变风险等问题,其应用范围仍然有限。相比之下,近些年发展起来的多种非基因工程细胞表面修饰技术,具有简便、易操作、适用范围广等优势,为调控细胞生命活动、赋予细胞新的性质和功能提供了更多新的选择,因而其在基础生物学研究和新药研发中具有广泛应用前景。对目前非基因工程技术的原理、特点以及在细胞治疗领域的应用等进行了综述和讨论,期望有助于深入了解这一新技术及其在生物医学领域中的应用。
中图分类号:
毕瑞, 武江波, 马春靖. 基于非基因工程技术的细胞表面修饰策略研究进展[J]. 生物技术进展, 2024, 14(4): 555-565.
Rui BI, Jiangbo WU, Chunjing MA. Research Progress on Cell Surface Modification Strategies Based on Non-genetic Engineering Technologies[J]. Current Biotechnology, 2024, 14(4): 555-565.
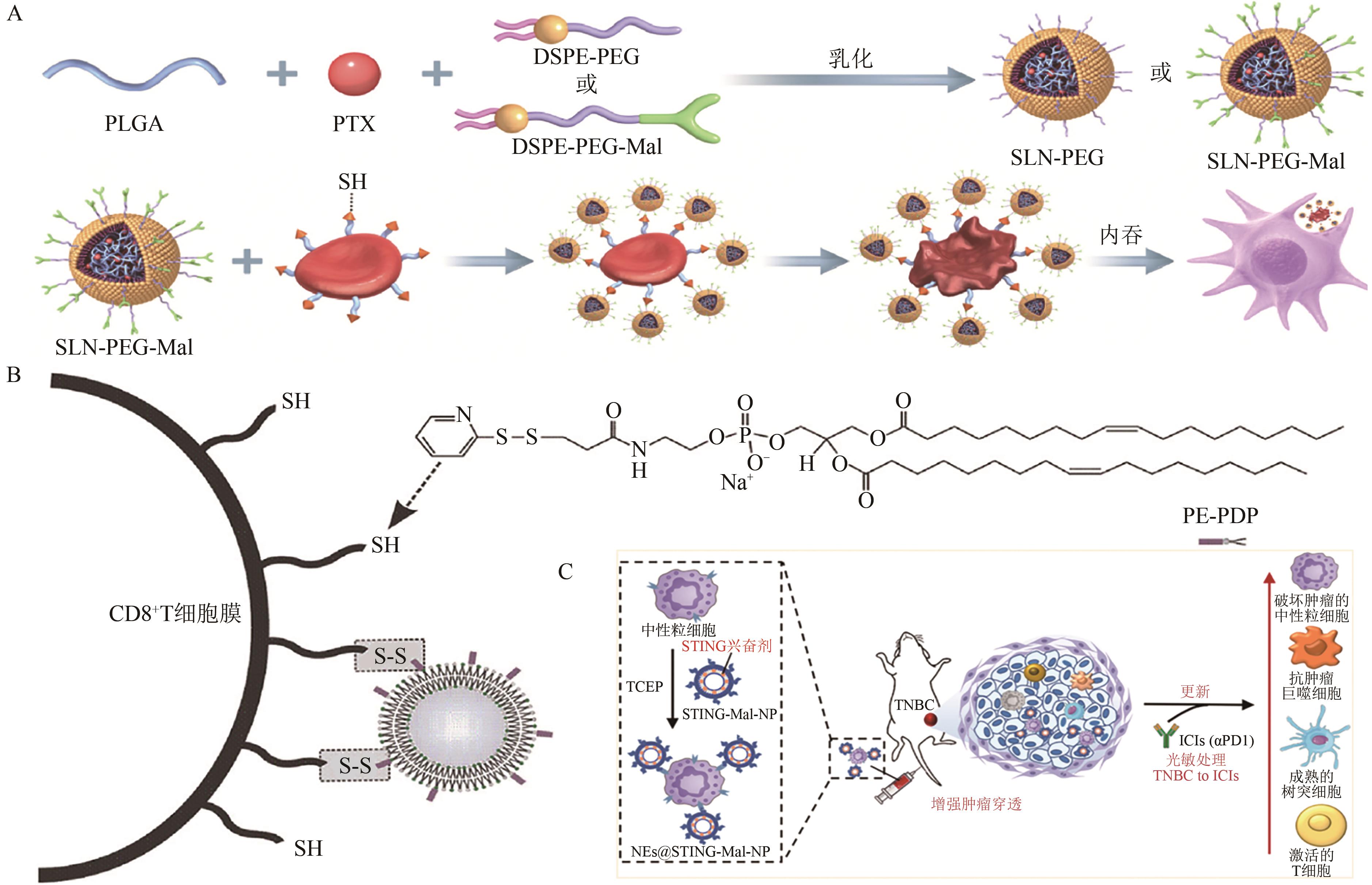
图2 基于巯基的修饰策略与应用A:PEG修饰纳米颗粒与红细胞表面巯基的共价偶联及其被巨噬细胞吞噬示意图[23];B:PDP介导的二硫键形成反应用于细胞表面装载脂质体颗粒示意图[26];C:NEs@STING-Mal-NP的制备和抗肿瘤原理示意图[25]。PLGA—聚乳酸-聚甘醇酸;PTX—紫杉醇;DSPE-PEG—1,2-二硬脂酰基-sn-丙三基-3-磷脂酰乙醇胺-聚乙二醇;Mal—马来酰亚胺;SLN—固体脂质纳米粒子;TAM—肿瘤相关巨噬细胞;MDSC—髓源性抑制性细胞;TCEP—三(2-羧乙基)膦;TNBC—三阴性乳腺癌;ICIs(aPD1)—免疫检查点抑制剂(抗程序性细胞死亡蛋白1)。
Fig. 2 Thiol-based chemical modification strategies and applications
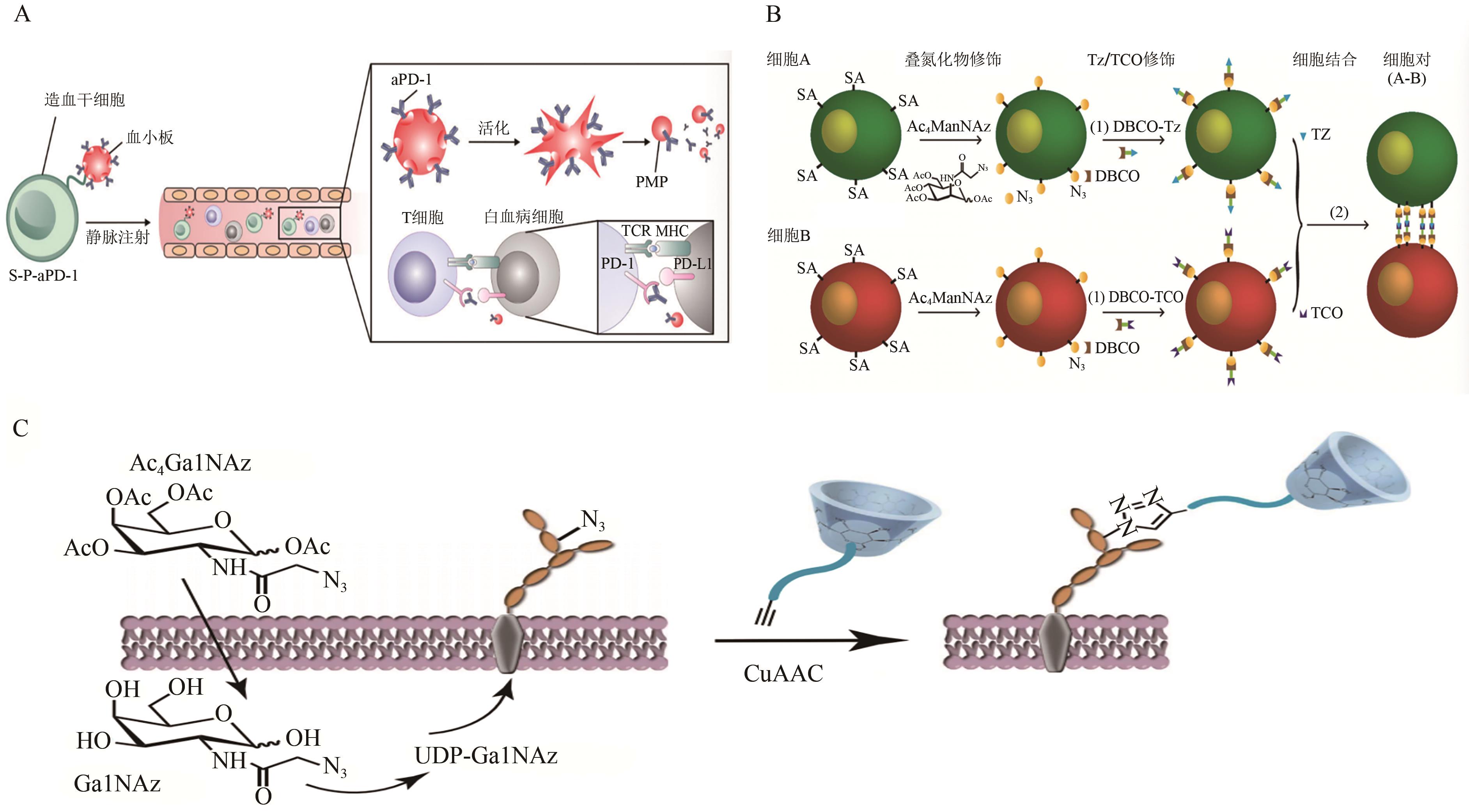
图3 代谢寡糖工程的应用A:造血干细胞通与血小板组装后辅助aPD-1递送示意图[36];B:应用代谢寡糖工程和双正交化学诱导细胞间粘附示意图[30];C:应用代谢标记法和生物正交点击反应将炔烃和PEG修饰的β-CD与细胞膜结合示意图[37]。SA—唾液酸;PMP—血小板微颗粒;TCR—T细胞受体;MCH—主要组织相容性复合体;DBCO—二苯并环辛炔;TCO—反式环辛烯;GalNAz—N-叠氮乙酰半乳糖胺;UDP-GalNAz—尿苷5'-二磷酸-N-叠氮乙酰半乳糖胺二钠盐。
Fig. 3 The application of metabolic oligosaccharide engineering.

图4 酶修饰的应用策略A:岩藻糖基转移酶辅助下将抗体偶联到细胞表面示意图[48];B:酪氨酸标记的纳米体在酪氨酸酶辅助下将蛋白质附着到细胞上的示意图[49];C:Herceptin-NK-92MI共轭物特异性结合HER2阳性癌细胞示意图[48];D:在Sortase A酶辅助下用携带GGG的抗原肽标记细胞的示意图[43]。ACC—抗体细胞偶联物;GDP—二磷酸鸟苷;GF-IgG—抗体与二磷酸鸟苷-岩藻糖偶联物;α1,3 FucT—α1,3岩藻糖基转移酶;abTRY—双孢蘑菇的酪氨酸酶;LPET—氨基酸序列,包括leucine(亮氨酸)、proline(脯氨酸)、glutamic acid(谷氨酸)、threonine(苏氨酸);GGG—三个甘氨酸。
Fig. 4 Application strategies of enzymatic modification
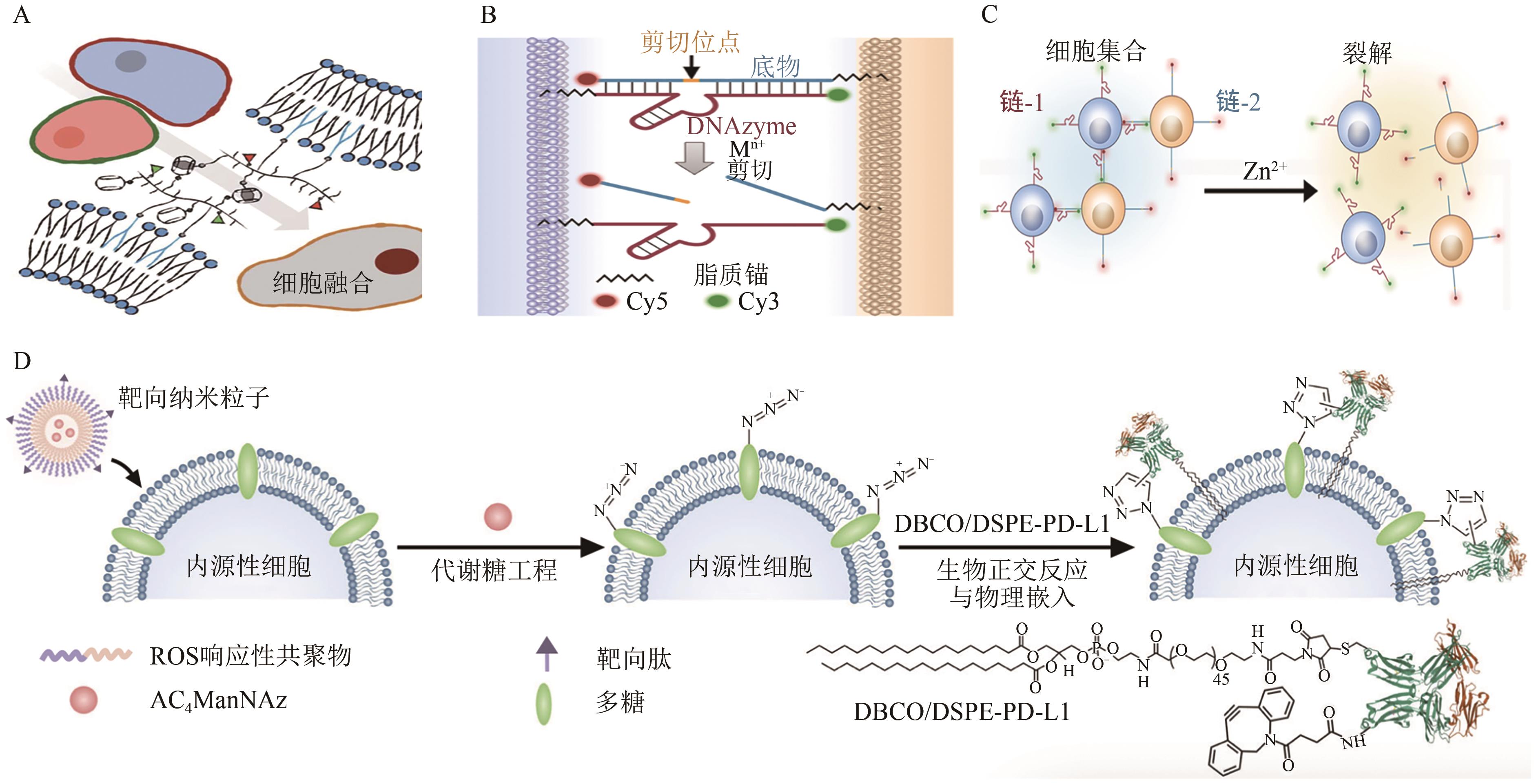
图5 基于疏水性插入修饰策略与应用A:基于疏水性插入策略下的细胞融合[63];B~C:DNAzyme与底物在特定金属离子辅助下实现了细胞之间的组装和解离[64];D:将PD-L1分子固定在靶向细胞膜上的示意图[65]
Fig. 5 Hydrophobic insertion-based modification strategies and applications
| 1 | ZHANG S Y, ZHOU Z R, QIAN R C. Recent progress and perspectives on cell surface modification[J]. Chem. Asian J., 2021, 16(21): 3250-3258. |
| 2 | SHI P, WANG Y. Synthetic DNA for cell-surface engineering[J]. Angew. Chem. Int. Ed., 2021, 60(21): 11580-11591. |
| 3 | LIU L, HE H, LIU J. Advances on non-genetic cell membrane engineering for biomedical applications[J/OL]. Polymers, 2019, 11(12): 2017[2024-05-29]. . |
| 4 | WEBER E W, MAUS M V, MACKALL C L. The emerging landscape of immune cell therapies[J]. Cell, 2020, 181(1): 46-62. |
| 5 | PRASAD V. Tisagenlecleucel-the first approved CAR-T-cell therapy: implications for payers and policy makers[J]. Nat. Rev. Clin. Oncol., 2018, 15(1): 11-12. |
| 6 | BONIFANT C L, JACKSON H J, BRENTJENS R J, et al.. Toxicity and management in CAR T-cell therapy[J/OL]. Mol. Ther. Oncolytics, 2016, 3: 16011[2024-05-29]. . |
| 7 | LI Z, HU S, CHENG K. Chemical engineering of cell therapy for heart diseases[J]. Acc. Chem. Res., 2019, 52(6): 1687-1696. |
| 8 | PATIL U S, QU H, CARUNTU D, et al.. Labeling primary amine groups in peptides and proteins with N-hydroxysuccinimidyl ester modified Fe3O4@SiO2 nanoparticles containing cleavable disulfide-bond linkers[J]. Bioconjug. Chem., 2013, 24(9): 1562-1569. |
| 9 | THOMSEN T, KLOK H A. Chemical cell surface modification and analysis of nanoparticle-modified living cells[J]. ACS Appl. Bio. Mater., 2021, 4(3): 2293-2306. |
| 10 | SARKAR D, SPENCER J A, PHILLIPS J A, et al.. Engineered cell homing[J]. Blood, 2011, 118(25): 184-191. |
| 11 | WANG W, GAN Q, ZHANG Y, et al.. Polymer-assisted metallization of mammalian cells[J/OL]. Adv. Mater., 2021, 33(34): e2102348[2024-06-04]. . |
| 12 | JASIEWICZ N E, MEI K C, OH H M, et al.. Zippercells exhibit enhanced accumulation and retention at the site of myocardial infarction[J/OL]. Adv. Healthc. Mater., 2023, 12(4): e2201094[2024-02-20]. . |
| 13 | WANG C, SUN X, CHENG L, et al.. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer[J]. Adv. Mater. 2014, 26(28): 4794-4802. |
| 14 | CAI F, REN Y, DAI J, et al.. Effects of various cell surface engineering reactions on the biological behavior of mammalian cells[J/OL]. Macromol. Biosci., 2023, 23(3): 2200379[2024-02-20]. . |
| 15 | MOORE C J, MONTÓN H, O'KENNEDY R, et al.. Controlling colloidal stability of silica nanoparticles during bioconjugation reactions with proteins and improving their longer-term stability, handling and storage[J]. J. Mater. Chem. B, 2015, 3(10): 2043-2055. |
| 16 | XU M, ASGHAR S, DAI S, et al.. Mesenchymal stem cells-curcumin loaded chitosan nanoparticles hybrid vectors for tumor-tropic therapy[J]. Int. J. Biol. Macromol., 2019, 134: 1002-1012. |
| 17 | CSIZMAR C M, PETERSBURG J R, WAGNER C R. Programming cell-cell interactions through non-genetic membrane engineering[J]. Cell Chem. Biol., 2018, 25(8): 931-940. |
| 18 | SUN X, HAN X, XU L, et al.. Surface-engineering of red blood cells as artificial antigen presenting cells promising for cancer immunotherapy[J/OL]. Small, 2017, 13(40): 1701864[2024-06-04]. . |
| 19 | SCOTT M D, MURAD K L, KOUMPOURAS F, et al.. Chemical camouflage of antigenic determinants: stealth erythrocytes[J]. Proc. Natl. Acad. Sci. USA, 1997, 94(14): 7566-7571. |
| 20 | TERAMURA Y, IWATA H. Islets surface modification prevents blood-mediated inflammatory responses[J]. Bioconjugate Chem., 2008, 19(7): 1389-1395. |
| 21 | RENAULT K, FREDY J W, RENARD P Y, et al.. Covalent modification of biomolecules through maleimide-based labeling strategies[J]. Bioconjugate Chem., 2018, 29(8): 2497-2513. |
| 22 | STEPHAN M T, MOON J J, UM S H, et al.. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles[J]. Nat. Med., 2010, 16(9): 1035-1041. |
| 23 | WANG X, MENG X, MAO K, et al.. Maleimide as the PEG end-group promotes macrophage-targeted drug delivery of PEGylated nanoparticles in vivo by enhancing interaction with circulating erythrocytes[J/OL]. Biomaterials, 2023, 300: 122187[2024-02-19]. . |
| 24 | LUO Z, LUO L, LU Y, et al.. Dual-binding nanoparticles improve the killing effect of T cells on solid tumor[J/OL]. J. Nanobiotechnol., 2022, 20(1): 261[2024-02-15]. . |
| 25 | HAO M, ZHU L, HOU S, et al.. Sensitizing tumors to immune checkpoint blockage via STING agonists delivered by tumor-penetrating neutrophil cytopharmaceuticals[J]. ACS Nano, 2023, 17(2): 1663-1680. |
| 26 | WAYTECK L, DEWITTE H, BACKER L D, et al.. Hitchhiking nanoparticles: reversible coupling of lipid-based nanoparticles to cytotoxic T lymphocytes[J]. Biomaterials, 2016, 77: 243-254. |
| 27 | LI Y, QIN H, YE M. An overview on enrichment methods for cell surface proteome profiling[J]. J. Sep. Sci., 2020, 43(1): 292-312. |
| 28 | MA N, HU J, ZHANG Z M, et al.. 2H-azirine-based reagents for chemoselective bioconjugation at carboxyl residues inside live cells[J]. J. Am. Chem. Soc., 2020, 142(13): 6051-6059. |
| 29 | DUBE D H, BERTOZZI C R. Metabolic oligosaccharide engineering as a tool for glycobiology[J]. Curr. Opin. Chem. Biol., 2003, 7(5): 616-625. |
| 30 | KOO H, CHOI M, KIM E, et al.. Bioorthogonal click chemistry-based synthetic cell glue[J]. Small., 2015, 11(48): 6458-6466. |
| 31 | AGATEMOR C, BUETTNER M J, ARISS R, et al.. Exploiting metabolic glycoengineering to advance healthcare[J]. Nat. Rev. Chem., 2019, 3(10): 605-620. |
| 32 | SAXON E, BERTOZZI C R. Cell surface engineering by a modified staudinger reaction[J]. Science, 2000, 287(5460): 2007-2010. |
| 33 | WRATIL P R, HORSTKORTE R, REUTTER W. Metabolic glycoengineering with N-acyl side chain modified mannosamines[J]. Angew. Chem. Int. Ed., 2016, 55(33): 9482-9512. |
| 34 | JACOBS C L, GOON S, YAREMA K J, et al.. Substrate specificity of the sialic acid biosynthetic pathway[J]. Biochemistry, 2001, 40(43): 12864-12874. |
| 35 | DOLD J E G A, PFOTZER J, SPÄTE A K, et al.. Dienophile-modified mannosamine derivatives for metabolic labeling of sialic acids: a comparative study[J]. ChemBioChem, 2017, 18(13): 1242-1250. |
| 36 | HU Q, SUN W, WANG J, et al.. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy[J]. Nat. Biomed. Eng., 2018, 2(11): 831-840. |
| 37 | SHI P, JU E, YAN Z, et al.. Spatiotemporal control of cell-cell reversible interactions using molecular engineering[J/OL]. Nat. Commun., 2016, 7: 13088[2024-02-19]. . |
| 38 | ZENG Y, RAMYA T N, DIRKSEN A, et al.. High-efficiency labeling of sialylated glycoproteins on living cells[J]. Nat. Meth., 2009, 6(3): 207-209. |
| 39 | DE BANK P A, KELLAM B, KENDALL D A, et al.. Surface engineering of living myoblasts via selective periodate oxidation[J]. Biotechnol. Bioeng., 2003, 81(7): 800-808. |
| 40 | SINCLAIR J, SALEM A K. Rapid localized cell trapping on biodegradable polymers using cell surface derivatization and microfluidic networking[J]. Biomaterials, 2006, 27(9): 2090-2094. |
| 41 | LIU X, WANG Y, YE B, et al.. Catalyst-free thiazolidine formation chemistry enables the facile construction of peptide/protein-cell conjugates (PCCs) at physiological pH[J]. Chem. Sci., 2023, 14(26): 7334-7345. |
| 42 | BI X, YIN J, CHEN GUANBANG A, et al.. Chemical and enzymatic strategies for bacterial and mammalian cell surface engineering[J]. Chemistry., 2018, 24(32): 8042-8050. |
| 43 | PISHESHA N, BILATE A M, WIBOWO M C, et al.. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease[J]. Proc. Natl. Acad. Sci. USA, 2017, 114(12): 3157-3162. |
| 44 | HONG S, SHI Y, WU NC, et al.. Bacterial glycosyltransferase-mediated cell-surface chemoenzymatic glycan modification[J/OL]. Nat. Commun., 2019, 10(1): 1799[2024-02-15]. . |
| 45 | FERNÁNDEZ-SUÁREZ M, BARUAH H, MARTÍNEZ-HERNÁNDEZ L, et al.. Redirecting lipoic acid ligase for cell surface protein labeling with small-molecule probes[J]. Nat. Biotechnol., 2007, 25(12): 1483-1487. |
| 46 | GEORGE N, PICK H, VOGEL H, et al.. Specific labeling of cell surface proteins with chemically diverse compounds[J]. J. Am. Chem. Soc., 2004, 126(29): 8896-8897. |
| 47 | HARMAND T J, PISHESHA N, REHM F B H, et al.. Asparaginyl ligase-catalyzed one-step cell surface modification of red blood cells[J]. ACS Chem. Biol., 2021, 16(7): 1201-1207. |
| 48 | LI J, CHEN M, LIU Z, et al.. A single-step chemoenzymatic reaction for the construction of antibody-cell conjugates[J]. ACS Cent. Sci., 2018, 4(12): 1633-1641. |
| 49 | MAZA J C, GARCÍA-ALMEDINA D M, BOIKE L E, et al.. Tyrosinase-mediated synthesis of nanobody-cell conjugates[J]. ACS Cent. Sci., 2022, 8(7): 955-962. |
| 50 | ALMARAZ R T, LI Y. Labeling glycans on living cells by a chemoenzymatic glycoengineering approach[J]. Biol. Open, 2017, 6(6): 923-927. |
| 51 | CAPICCIOTTI C J, ZONG C, SHEIKH M O, et al.. Cell-surface glyco-engineering by exogenous enzymatic transfer using a bifunctional CMP-Neu5Ac derivative[J]. J. Am. Chem. Soc., 2017, 139(38): 13342-13348. |
| 52 | ABUKAR T, RAHMANI S, THOMPSON N K,et al.. Development of BODIPY labelled sialic acids as sialyltransferase substrates for direct detection of terminal galactose on N- and O-linked glycans[J/OL]. Carbohydr. Res., 2021, 500: 108249[2024-02-15]. . |
| 53 | SUN T, YU S H, ZHAO P, et al.. One-step selective exoenzymatic labeling (SEEL) strategy for the biotinylation and identification of glycoproteins of living cells[J]. J. Am. Chem. Soc., 2016, 138(36): 11575-11582. |
| 54 | VARGAS-RODRIGUEZ O, SEVOSTYANOVA A, SÖLL D, et al.. Upgrading aminoacyl-tRNA synthetases for genetic code expansion[J]. Curr. Opin. Chem. Biol., 2018, 46: 115-122. |
| 55 | HOEHNEL S, LUTOLF M P. Capturing cell-cell interactions via SNAP-tag and CLIP-tag technology[J]. Bioconjugate Chem., 2015, 26(8): 1678-1686. |
| 56 | KUMARI P, BOWMIK S, PAUL S K, et al.. Sortase A: a chemoenzymatic approach for the labeling of cell surfaces[J]. Biotechnol. Bioeng., 2021, 118(12): 4577-4589. |
| 57 | RAMYA T N, WEERAPANA E, CRAVATT B F, et al.. Glycoproteomics enabled by tagging sialic acid- or galactose-terminated glycans[J]. Glycobiology, 2013, 23(2): 211-221. |
| 58 | MATTEY A, BIRMINGHAM W R, BOTH P, et al.. Selective oxidation of N-glycolylneuraminic acid using an engineered galactose oxidase variant[J]. ACS Catal., 2019, 9(9): 8208-8212. |
| 59 | WON Y W, PATEL A N, BULL D A. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient[J]. Biomaterials, 2014, 35(21): 5627-5635. |
| 60 | DAVIS K A, WU P J, CAHALL C F, et al.. Coatings on mammalian cells: interfacing cells with their environment[J/OL]. J. Biol. Eng., 2019, 13: 5[2024-02-25]. . |
| 61 | GABRIELSE K, GANGAR A, KUMAR N, et al.. Reversible re-programing of cell-cell interactions[J]. Angew. Chem. Int. Ed., 2014, 53(20): 5112-5116. |
| 62 | HAO M, HOU S, Li W, et al.. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy [J/OL]. Sci. Transl. Med., 2020, 12(571): eaaz6667[2024-02-28]. . |
| 63 | HUANG F, LIU J, LIU Y. Engineering living cells with cucurbit[7]uril-based supramolecular polymer chemistry: from cell surface engineering to manipulation of subcellular organelles[J]. Chem. Sci., 2022, 13(30): 8885-8894. |
| 64 | QIAN R C, ZHOU Z R, GUO W, et al.. Cell surface engineering using DNAzymes: metal ion mediated control of cell-cell interactions[J]. J. Am. Chem. Soc., 2021, 143(15): 5737-5744. |
| 65 | WANG S, ZHANG Y, WANG Y, et al.. An in situ dual-anchoring strategy for enhanced immobilization of PD-L1 to treat autoimmune diseases[J/OL]. Nat. Commun., 2023, 14(1): 6953[2024-02-15]. . |
| 66 | UVYN A, COEN R D, GRUIJS M, et al.. Efficient innate immune killing of cancer cells triggered by cell-surface anchoring of multivalent antibody-recruiting polymers[J]. Angew. Chem. Int. Ed., 2019, 58(37): 12988-12993. |
| 67 | GASPAR V M, LAVRADOR P, BORGES J, et al.. Advanced bottom-up engineering of living architectures[J/OL]. Adv. Mater., 2020, 32(6): e1903975[2024-02-15]. . |
| 68 | GAO W, XIAO Y. Advances in cell membrane-encapsulated biomaterials for tissue repair and regeneration[J/OL]. Appl. Mater. Today, 2022, 26: 101389[2024-06-04]. . |
| 69 | LEE D Y, CHA B H, JUNG M, et al.. Cell surface engineering and application in cell delivery to heart diseases[J/OL]. J. Biol. Eng., 2018, 12: 28[2024-02-15]. . |
| 70 | DUTTA D, PULSIPHER A, LUO W, et al.. Synthetic chemoselective rewiring of cell surfaces: generation of three-dimensional tissue structures[J]. J. Am. Chem. Soc., 2011, 133(22): 8704-8713. |
| 71 | ROGOZHNIKOV D, LUO W, ELAHIPANAH S, et al.. Generation of a scaffold-free three-dimensional liver tissue via a rapid cell-to-cell click assembly process[J]. Bioconjugate Chem., 2016, 27(9): 1991-1998. |
| 72 | ROGOZHNIKOV D, O'BRIEN P J, ELAHIPANAH S, et al.. Scaffold free bio-orthogonal assembly of 3-dimensional cardiac tissue via cell surface engineering[J/OL]. Sci. Rep., 2016, 6: 39806[2024-02-15]. . |
| 73 | LIN M, CHEN Y, ZHAO S, TANG R, et al.. A biomimetic approach for spatially controlled cell membrane engineering using fusogenic spherical nucleic acid[J/OL]. Angew. Chem. Int. Ed. Engl., 2022, 61(1): e202111647[2024-02-09]. . |
| 74 | KIM S, KIM K. Lipid-mediated ex vivo cell surface engineering for augmented cellular functionalities[J/OL]. Biomater. Adv., 2022, 140: 213059[2024-02-09]. . |
| 75 | JAHROMI L P, SHAHBAZI M A, MALEKI A, et al.. Chemically engineered immune cell-derived microrobots and biomimetic nanoparticles: emerging biodiagnostic and therapeutic tools[J/OL]. Adv. Sci., 2021, 8(8): 2002499[2024-02-15]. . |
| [1] | 张爽,郑冬,马月辉. 脂肪干细胞在再生医学中的应用[J]. 生物技术进展, 2015, 5(4): 291-296. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 2021《生物技术进展》编辑部